Template Original Research Template Case Report Guide for Author Online Submission
Editorial Policies
Focus and Scope
The journal encourages submissions from scholar practitioners, and professionals who are contributing to Medical Innovations and Technologies, Clinical Practices and Patient Care, Healthcare Management and Policy, Ethical Issues in Healthcare, and Healthcare Informatics and Telemedicine, and Excellent service related to Health Prevention and Promotion, especially in Healthcare services.
Peer Review Policy
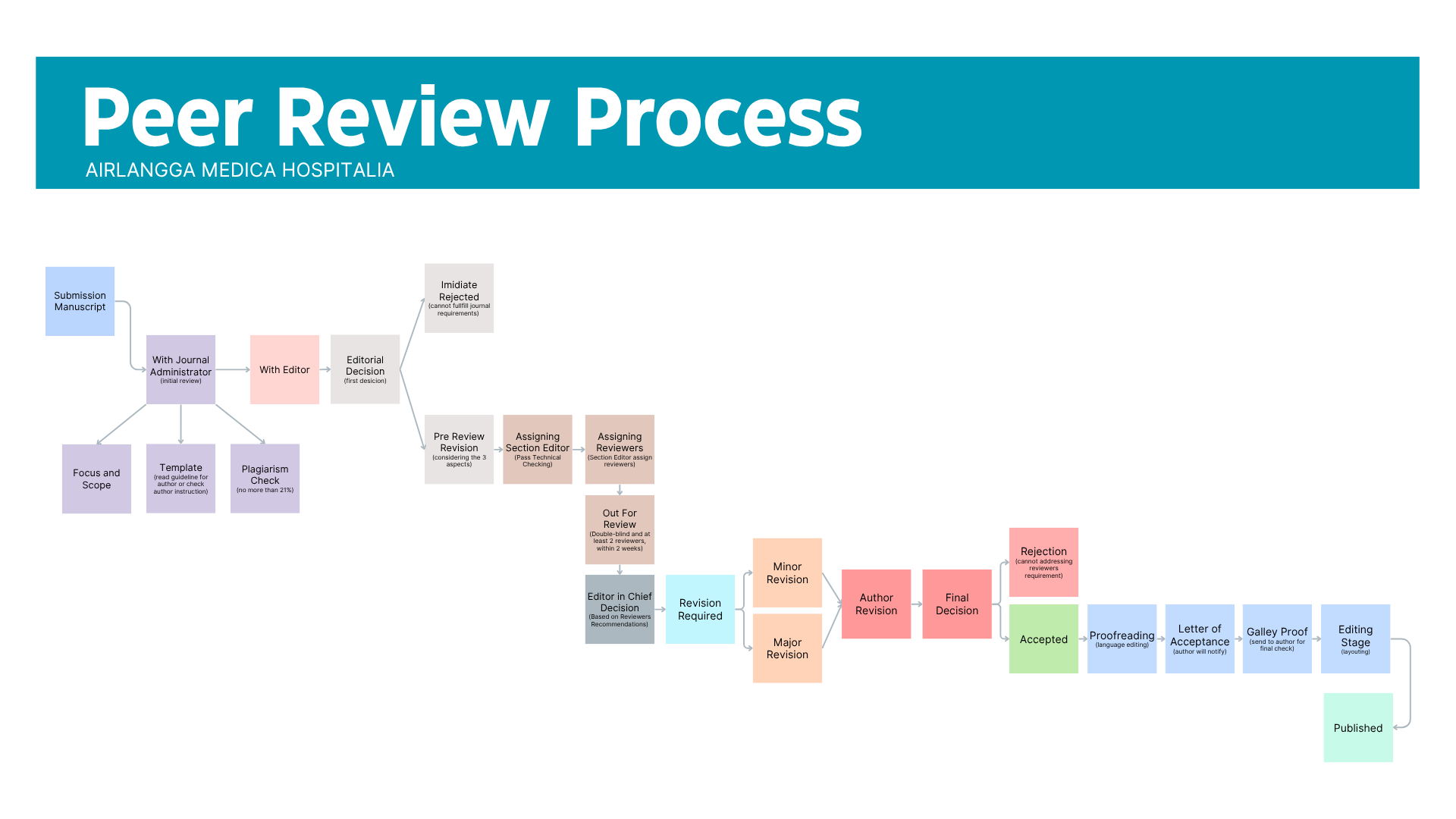
Submitted manuscripts will be pre-reviewed by the editors (initial review or desk review) regarding technical checking, including plagiarism and manuscript guidelines, to determine whether the manuscripts conform to the Journal of Airlangga Medica Hospitalia (AMH) submission guidelines. After passing the technical check, the article will be carried out through a double-blind peer review process by considering the substantial aspect in Medical Innovations and Technologies, Clinical Practices and Patient Care, Healthcare Management and Policy, Ethical Issues in Healthcare, and Healthcare Informatics and Telemedicine. Editors and reviewers provide constructive feedback on the evaluation results to the author(s). The Editor-in-Chief of AMH has the right and authority to decide which manuscripts submitted to the journal are considered for publication.
Peer review is responsible for critiquing by reading and evaluating a manuscript based on the reviewer's expertise, then giving constructive and objective feedback to the author. Manuscript.
Before reviewing, please follow these requirements.
- Review Invitation: Is the article requested to be reviewed following your expertise? If you receive a review invitation that covers topics that are not your expertise, please notify the journal as soon as possible and recommend an alternative reviewer in line with the topic and expertise.
- Timeline to Review: Do you have the time to review the article? The review process must be completed within two weeks. If you agree and require a longer period, notify the editors as soon as possible, or suggest an alternative reviewer.
When reviewers accept the invitation to review, please consider the following:
- Title: Is it illustrating the article?
- Abstract: does reflect the contents of the article
- Introduction: Does it describe the relevant previous studies, state of the art, novelty, and research gap?
To determine the originality of the journal, are there aspects of plagiarism over 20% of this paper field? A quick literature search can use certain tools such as Google Scholar, Scopus, PubMed, and WOS to see if there are similarities with other parts.
- Does the author accurately describe how the data is collected?
- Does the author provide the research methods, research design, and approach?
- Does the author provide research scope and settings
- Does the article identify the following procedures
This is where the author must explain the findings of the research. It should be laid out and in a logical sequence, including research findings, formulation, theoretical elaboration, data analysis, and interpretation.
- Does the author provide the practical or theoretical aspects of these results?
- How does the researcher explain any discrepancies compared to other studies?
- Does the author conclude from the results and discussion?
- Does the conclusion support the information and arguments presented?
- What are the implications of the conclusion for the readers?
- Does the conclusion suggest any actions or recommendations?
- Does the table accurately represent the data?
- Does the figure accurately represent the data?
- Does the table understandable to readers?
- Does the figure understandable to readers?
All submitted manuscripts are read by the Editor in Chief and the Editorial Board of the Airlangga Medica Hospitalia (AMH) initially for desk evaluation. An unsuitable submitted manuscript in terms of focus and scope would be rejected promptly without going through the peer review process. Manuscript evaluated to be of potential interest to our readership, and after passing the technical checking, would be sent to peer review. The editorial team then makes a decision based on the reviewer's recommendation from among several possibilities consider rejection, major revision required, minor revision, or accepted.
Reviewers will ask to check the manuscript following the main content such as introduction (previous studies, novelty state of the arts, and research gap), research methodology including approach, research instrument, and data collection, results and discussion (research findings and elaboration of theory), conclusion (summarizing and generalizing the main content)
The manuscript process will be to go through several steps, including 2 weeks for the first decision consists of technical checking and plagiarism checking. And then takes 4 – 12 weeks for the review process, consisting of review (round 1, round 2 or more) and revision required.
Publishing Frequency
Two times a year (Biannually) – June and November
Open Access Policy
The journal allows readers to read, download, copy, distribute, print, search, or link to the full texts of its articles and allows readers to use them for any other lawful purpose.
This journal provides immediate open access to its content on the principle that making research freely available to the public supports a greater global exchange of knowledge.
Airlangga Medica Hospitalia by Unair is licensed under a The Creative Commons Attribution license (CC BY).
Archiving
Airlangga Medica Hospitalia (AMH) follows the archiving policies established by Universitas Airlangga. Universitas Airlangga collaborates with Clockss to archive all journal files within its repository. According to these policies (2024), journals that have not yet been indexed by DOAJ will not be archived in Clockss. Therefore, Airlangga Medica Hospitalia (AMH) independently archives its manuscripts in the repositories of Universitas Airlangga (Library). Once AMH is indexed by DOAJ, Universitas Airlangga (Library) will archive the journal files in Clockss.
Publication Ethics
We adhere rigorously to its Code of Conduct and Best Practice Guidelines.
AMH maintains a stringent peer-review procedure with explicit ethical principles and standards to facilitate the inclusion of high-quality scientific research in scholarly publishing. Upon recognizing ethical issues, we are committed to conducting thorough investigations and implementing necessary measures to maintain the integrity of the literature and protect study participants.
PUBLICATION ETHICS AND MALPRACTICE STATEMENT
At Airlangga Medica Hospitalia, we are committed to maintaining the utmost standards of publication ethics to cultivate a responsible and ethical research atmosphere. We are dedicated to sharing valuable knowledge while upholding integrity, transparency, and ethical standards at every stage of our publication process. We have crafted detailed publication ethics guidelines that align with the principles established by the Committee on Publication Ethics (COPE) to guarantee this commitment. Airlangga Medica Hospitalia is committed to upholding the highest standards of publication ethics, fostering a responsible and ethical research environment. Our dedication lies in sharing valuable knowledge while maintaining the highest standards of integrity, transparency, and ethical conduct throughout the publication process. We have crafted thorough publication ethics guidelines that are in harmony with the principles set forth by the Committee on Publication Ethics (COPE).
1. Authorship
The concept of authorship and its accompanying responsibilities constitute the basis of ethical publishing practices. Authorship should be determined by significant contributions to the research. Authors must adhere to specific criteria, as established by COPE, to ensure appropriate credit is assigned. All authors listed must have made substantial intellectual contributions to the study. The corresponding author must ensure that all co-authors have reviewed and approved the final manuscript version prior to submission. Authors must provide precise affiliations, contact details, and disclose any potential conflicts of interest that could impact the integrity of the study.
2. Misconduct
Research Misconduct: In cases where there is suspicion of unethical research conduct, the Editor may reject the manuscript and notify pertinent third parties, including the author's institution and ethics committee. Demonstrated misconduct in published articles or substantial violations of scientific integrity may result in retraction.
Data Falsification and Fabrication: Data falsification entails the manipulation of data to provide a misleading representation, including the alteration of pictures or the exclusion of outliers. Data falsification entails the creation of fictitious study results. Concerns about data integrity during or post-peer review will be sent to the Editor, who may solicit anonymised underlying data for validation. Inability to provide original data may lead to rejection or retraction, with alleged misbehavior reported to the author's institution.
Publication Misconduct: Airlangga Medica Hospitalia follows COPE guideline for handling the publication misconduct
Duplicate Publication: Authors are required to disclose any potential overlaps or duplications in their work. All potentially overlapping publications must be disclosed and properly cited. Manuscripts must not have been formally published in other venues. Upon request, 'in press' or unpublished manuscripts must be provided to the Editor. AMH retains the authority to evaluate potential overlaps individually.
All research pieces must reference relevant literature to substantiate their assertions. Unacceptable citation methods, including excessive self-citation, collusive self-citation among authors, and superfluous mention of the journal to which the manuscript is submitted, are prohibited.
The Plagiarism Policy emphasises the importance of originality and the necessity of avoiding plagiarism as fundamental components of ethical publishing. AMH mandates that all submitted manuscripts be original and not previously published or currently under consideration in other venues. To prevent plagiarism, authors are advised to properly cite and reference others' work and utilise plagiarism detection software throughout the review process. The principles of originality and plagiarism avoidance are essential components of ethical publishing. In AMH, we emphasise that all submitted manuscripts must be original and not previously published or under consideration in other venues. To prevent plagiarism, authors are encouraged to accurately cite and reference the works of others and to employ plagiarism detection software during the review process.
3. Ethical Adherence
Research that involves human participants, human materials, or human data must comply with the guidelines established in the Declaration of Helsinki and obtain approval from an appropriate ethics committee. Research ethics and informed consent are essential in studies involving human and animal subjects. Authors must comply with ethical guidelines set forth by the Declaration of Helsinki or other pertinent standards, ensuring that informed consent has been appropriately obtained and that research has received approval from institutional review boards. Authors should disclose any potential conflicts of interest that may affect the research or its reporting.
In research involving animal experimentation, it is imperative for authors to rigorously assess the potential harm to the animals involved. This involves a detailed examination of the specific procedures and experiments, along with the number and species of animals used. Compliance of the animals involved in the experiments with institutional and national guidelines is essential. The manuscripts must clearly indicate that all feasible measures were implemented to reduce animal suffering.
In research involving animal experiments, authors must rigorously evaluate the potential harm to the animals involved. This encompasses a detailed analysis of the particular procedures and experiments performed, along with the quantity and species of animals utilized. The animals participating in the experiments must comply with institutional and national guidelines. Additionally, the manuscripts must explicitly indicate that all required measures were implemented to reduce animal suffering. All research involving human subjects requires obtaining informed consent from participants, or from their parents or legal guardians if the participants are minors under 18. A statement confirming this should be included in the manuscript. Manuscripts involving vulnerable populations, such as unconscious patients or prisoners, or where consent may lack full informativity, will be assessed at the editor's discretion. Such instances may necessitate additional review by an internal editorial oversight committee. Consent is necessary for all forms of personally identifiable information, encompassing biomedical, clinical, and biometric data. In studies concerning human transplantation, authors are required to confirm that no organs or tissues were obtained from prisoners and to specify the institutions, clinics, or departments involved in the procurement of these organs or tissues. Authors may be required to submit documentary evidence of consent upon request.
DUTIES OF AUTHORS
1. Reporting Standards
Authors must provide a precise representation of the actual study conducted and an impartial analysis of its importance. Authors must disclose their findings with integrity, devoid of fabrication, falsification, or improper data manipulation. A paper must provide enough material and citations to enable others to reproduce the research. Deceptive or willfully erroneous remarks represent unethical conduct.
2. Originality and Plagiarism
Authors must guarantee that their work is wholly unique. All use of others' work whether it data, writing, or concepts, must be appropriately referenced. Plagiarism, in all its forms, including self-plagiarism, data fabrication, or falsification, is deemed immoral and intolerable.
3. Authorship and Contribution
Only those who have substantially contributed to the idea, design, implementation, or interpretation of the research should be acknowledged as authors. Individuals who participated in small capacities (e.g., technical assistance, proofreading) should be duly thanked. All co-authors must concur on the final article version and its submission for publication.
4. Data Integrity and Transparency
Authors have the responsibility of providing precise data and outcomes. Original data must be retained and accessible for verification if necessary. Manipulation, selective reporting, or distortion of results erodes scientific credibility.
5. Acknowledgement of Sources
All sources and influences used in the study must be properly acknowledged. This includes referencing prior research, funding organizations, and any institutional or individual assistance.
6. Avoidance of Multiple or Redundant Publications
Authors are prohibited from publishing the same paper or substantially comparable material in several journals or main publications. Duplicate submission or duplicate publishing is unethical and depletes editorial and peer-review resources.
7. Conflict of Interest Disclosure
Authors must state any financial, institutional, or personal affiliations that may affect, or be seen to influence, their study. Transparency sustains confidence in the publication's neutrality and integrity.
8. Ethical Standards in Research
Authors must ensure adherence to ethical standards and relevant legislation when the study involves human participants, animals, or sensitive data. This includes acquiring informed permission, securing ethical approval, and complying with welfare regulations.
9. Correction of Errors
Authors must quickly inform the journal editor of any substantial mistakes or inconsistencies in their published work and collaborate in rectifying or withdrawing the manuscript. Accountability persists post-publication.
10. Respect for the Peer-Review Process
Authors are required to address reviewers' remarks constructively and expeditiously. Revisions must be conducted meticulously, and authors should refrain from inappropriately influencing the review process.
DUTIES OF EDITORS
1. Fairness and Impartiality in Decision-Making
Editors are required to assess submitted papers only on the grounds of academic excellence, originality, and relevance to the journal's scope, without bias regarding the author's race, gender, country, institutional affiliation, or political/religious convictions.
2. Confidentiality
Editors must maintain the confidentiality of information pertaining to submitted papers. Manuscripts must not be disseminated beyond the editorial board, reviewers, and publisher without the author's consent.
3. Handling of Conflicts of Interest
Editors must prevent circumstances in which conflicts of interest might compromise editorial judgment. If conflicts arise, the editor must recuse themselves from managing the manuscript.
4. Handling of Conflicts of Interest
Editors must uphold openness in their decision-making processes by explicitly conveying acceptance, modification, or rejection outcomes to writers, accompanied by constructive criticism. They are tasked for guaranteeing the publication of only high-quality, ethically conducted research.
5. Ensuring Integrity of the Academic Record
Editors must ensure the precision and dependability of published material. Upon the discovery of mistakes, misleading information, or misbehavior, editors must provide corrections, retractions, or expressions of concern in accordance with COPE principles.
6. Peer Review Oversight
Editors are tasked with orchestrating a fair, impartial, and prompt peer-review process. They must guarantee that reviewers possess the requisite competence, are chosen judiciously, and provide assessments devoid of personal prejudice.
7. Dealing with Misconduct
Editors must use appropriate measures to detect and prevent publishing misconduct, including plagiarism, data falsification, or unethical research practices. Upon suspicion of misbehavior, an investigation must be conducted, and necessary measures taken in accordance with COPE flowcharts.
8. Commitment to Academic Freedom and Ethical Standards
Editors must advocate for appropriate research methodologies, adhere to ethical standards consistent with international norms, and foster academic freedom while preserving the integrity of the publishing process.
DUTIES OF REVIEWERS
1. Contribution to Editorial Decision
Reviewers provide constructive, impartial, and evidence-based evaluations that aid editors in determining article acceptance, amendment, or rejection. Their function is to enhance the quality of disseminated research.
2. Promptness and Responsibility
Reviewers must promptly reply to invites and deny review requests if they believe they are unqualified or unable to complete the review within the stipulated period.
3. Confidentiality
Manuscripts undergoing review are confidential materials. Reviewers are prohibited from sharing, discussing, or using any material from a manuscript for personal gain or with others without prior consent from the editor.
4. Objectivity and Fairness
Reviews must be executed impartially, devoid of personal disparagement towards the author. Critiques should be founded on academic reasoning, empirical data, and relevance, rather than prejudice or personal convictions.
5. Acknowledgment of Sources
Reviewers must discover pertinent published works that the authors have not mentioned. Should they see significant resemblance or overlap between the article under review and other published or submitted works, they are obligated to inform the editor.
6. Conflict of Interest
Reviewers are required to disclose any possible conflicts of interest (financial, institutional, collaborative, or personal affiliations) that may affect their evaluation. If such conflicts are present, they should refuse the review.
7. Ethical Oversight
Reviewers who suspect ethical violations, including plagiarism, data falsification, or research misconduct, must notify the editor with substantiated proof.
8. Respect for Anonymity
In double-blind peer review processes, reviewers are obligated to maintain the anonymity of authors and refrain from attempting to identify them.
GENERATIVE ARTIFICIAL INTELLIGENCE (AI) POLICY
Airlangga Medica Hospitalia (AMH) upholds the highest standards of academic integrity, transparency, and ethical publishing in accordance with the guidelines set by the Committee on Publication Ethics (COPE). In response to the increasing use of Generative Artificial Intelligence (AI) tools (e.g., ChatGPT, Claude, Grammarly, Quilbot, etc.) in scholarly communication, AMH has established the following policy to ensure responsible, transparent, and ethical use of AI technology throughout the publication process.
1. For Author
"AI tools cannot meet the requirement for authorship as they cannot take responsibility for the submitted work. Authors who use AI tools must be transparent in disclosure, how the AI tools was used, and which tool was used. Authors are fully responsible for the content of their manuscript, even those parts produced by an AI tool, and are thus liable for any breach of publication ethics" (COPE, 2023, paras. 1 & 2)
The author may use generative artificial intelligence (AI) and AI-assisted technology, such as language models, translation tools, or grammar correction, provided that such use is conducted responsibly and transparently. However, AI tools must not be listed as the author and cannot take responsibility for the integrity, originality, or accuracy of the manuscript. The authors bear full accountability for all content generated with the assistance of AI systems. Any text, image, data, or idea produced wholly or partially through generative AI tools must be critically reviewed, verified, and appropriately attributed by the authors.
The editorial board encourages authors to remain cautious of potential biases, inaccuracies, and data privacy concern that may arise from AI tools and to ensure that their use complies with institutional, ethical, and legal standards. Author remain fully responsible for ensuring that the final version of the manuscript is accurate, original, and aligned with the principles of research and publication ethics
2. For Peer Reviewer
“Reviewers should respect the confidentiality of the manuscripts they evaluate. They should not disclose any information about the work or use it for personal advantage, ensuring the integrity of the review process” (The Ethical Guidelines for Peer Reviewers)
During the peer review process, no generative AI or AI-assisted technologies were used to generate, interpret, or evaluate the content of the manuscript. The review was conducted independently by the reviewers to ensure the integrity, confidentiality, and objectivity of the assessment in accordance with COPE Guidelines
If any AI-assisted tools were used, they were limited to non-interpretative functions such as grammar correction, language clarity, or reference formatting. The reviewers confirms that no part of the critical evaluation, judgement, or decision-making was delegated to generative AI systems.
3. For Editors
“…..The use of AI is implicated in this case, they could present it to the author in terms of their policies on transparency. For example, the COPE policy state any used of generative AI in manuscript preparation should be declared or needs to be described” (Advice from COPE Council, under the case number 25-01, 2025)
Editors, editorial board member, and staf involved in the management of the journal must uphold transparency and integrity when using generative AI or AI-assisted technologies during the editorial process. The use of such tools should be limited to supporting administrative and technical tasks, such as plagiarism detection, language editing, or workflow management, and must not replace critical human judgment in editorial decision-making. If generative AI tools (e.g. ChatGPT, GrammarlyGO, or similar systems) are used to assist in drafting communication, improving readability, or summarizing content, editors must ensure that all outputs are reviewed and validated by a human before dissemination. Editors are required to disclose in the editorial records or documentation of AI-assisted tools were used in any substantive part of the decision-making process or communication with authors and reviewers. Editors must not upload unpublished manuscripts, reviewers report, or confidential material into generative AI systems that store data externally, to avoid breaches of confidentiality and data protection standards. All editorial decisions must remain human-driven, based on ethical publishing guidelines and the journal’s established review policies.
RETRACTING AND WITHDRAWAL POLICY
In general, journal editors do not have the autonomy to determine which articles will be published. When it comes to publishing decisions, editors follow the guidelines set by the journal's editorial board and must adhere to relevant legal standards concerning defamation, copyright infringement, duplicate publishing, authorship matters, and plagiarism. Published articles will remain available, precise, and unchanged to the best of our ability. However, certain circumstances can arise in which published articles must be withdrawn or even deleted. Such actions ought to be undertaken only in extraordinary situations. Journal editors, authors, and/or their institutions can initiate a retraction of published articles. In some instances, the retraction should include an apology for the earlier mistakes and/or expressions of gratitude to those who brought the error to the author's attention. A retraction of published scientific articles should include a statement indicating that the original article must not be published and that the data and conclusions should not serve as a foundation for future research.
1. Article Withdrawal
This situation may arise if the original version of the manuscript has an error or if it was inadvertently submitted to both AMH and/or another publisher more than once. Moreover, it may arise from violations of the scientific code of ethics, including double submissions, authorship disputes, plagiarism, self-plagiarism, fraudulent data usage, or similar concerns. Articles that violate the code of ethics, when the author is aware, may be withdrawn by the author through a formal letter addressed to the editorial board of AMH.
2. Article Retraction
If an article is found to have violated scientific ethical regulations, such as plagiarism, fraudulent use of data, authorship issues, double submissions, or the use of false authors, a retraction is issued. Additionally, a retraction will be implemented to rectify errors that occurred during the submission or publication process. The editorial council of the AMH recommends that the author or editor retract an article.
Copyrights Notice
Airlangga Medica Hospitalia by Unair is licensed under a The Creative Commons Attribution license (CC BY).
- Every manuscript submitted must observe the policy and terms set by Airlangga Medica Hospitalia.
- Publication rights to the manuscript content published by Airlangga Medica Hospitalia is owned by the journal with the consent and approval of the author(s) concerned.
- Full texts of electronically published manuscripts can be accessed free of charge and used according to the license shown above.
Author Fee
All articles submitted to AMH will incur No Article Processing Charges. This encompasses submission, peer review, editing, publication, maintenance, archiving, and facilitates rapid access to the full text versions of the articles.
Plagiarism Checking
|
Similarity rate |
Description |
|
10% - 15% |
The manuscript may pass the initial review (desk review) |
|
16% - 20% |
The manuscript will be sent back to the author for major correction and improvement – the author is required to paraphrase properly |
|
>21% |
The manuscript does not pass the initial review and must be declined at the editorial stage (reject on desk review) |
Turnitin Plagiarism Check (https://www.turnitin.com/)






