Early Sex Differentiation of Climbing Perch (Anabas testudineus Bloch.): A Pathway to Feminization
Downloads
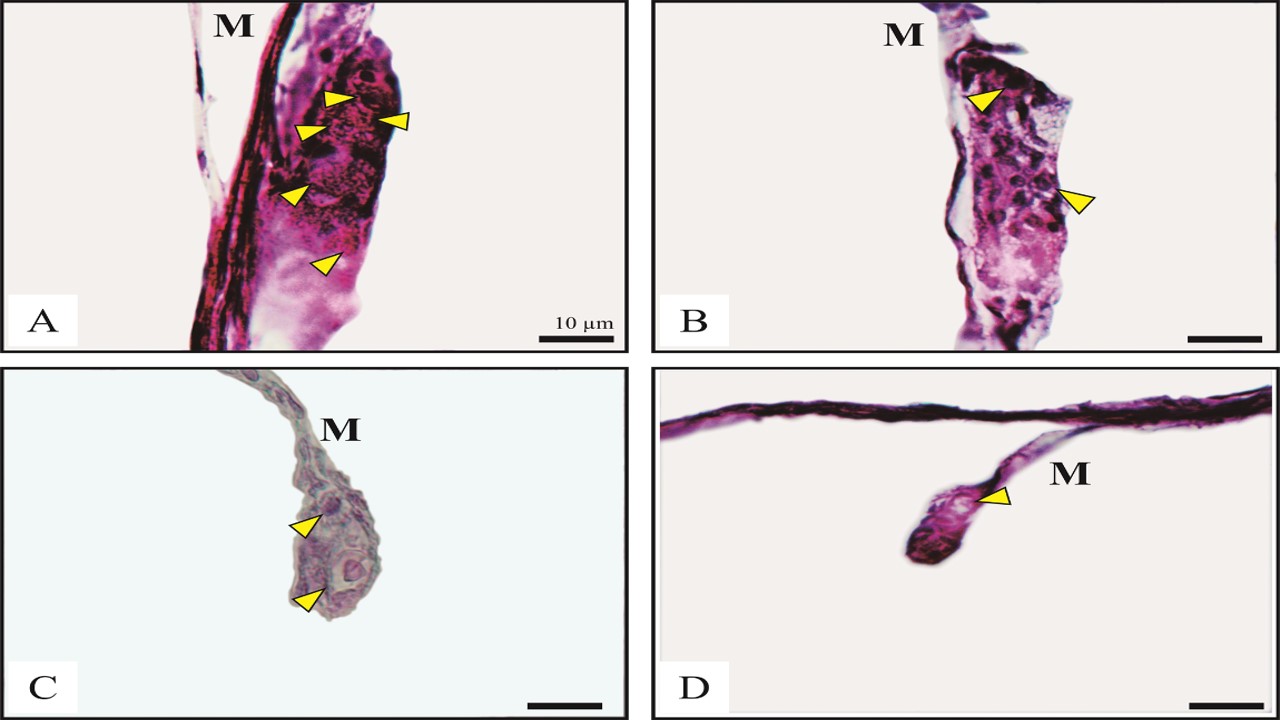
The phenomenon of sexual dimorphism in climbing perch, which shows that female fish grow faster than males, underlies the development of mono-sex culture. Female mono-sex culture is more applicable for farmers by crossing neo-male fish with normal females. The timing of sexual differentiation in climbing perch is still unknown. It is very useful in sex reversal procedures to produce neo-male climbing perch. This study revealed the time and status of climbing perch sexual differentiation. Ten samples of climbing perch from the spawning of five pairs of parents were taken from the nursery pond at 10–29 days post-hatching (dph). Samples were prepared through a histology preparation procedure. Observations of the structure and characteristics of the gonads were carried out using a light microscope and analyzed histologically. The results indicated that gonad samples aged 10, 11, 12, 13, 15, and 16 dph showed primordial germ cells surrounded by somatic tissue forming genital ridges and mitotic division. Meanwhile, the gonads begin to differentiate as ovaries found at 18 dph with the presence of oogonia and ovarian cavities. Gonads aged 20–21 dph increasingly showed single oogonia cells (size 20–37.5 µm), germ cell cysts, genital ridges, oocytes undergoing the vitellogenesis process, perinucleolar oocytes, and the formation of the ovarian cavity. Sex differentiation of climbing perch was predicted from 18–21 dph. This conclusion underlies that the sex reversal procedure in climbing perch must be carried out before 18 dph.
Ahmad, A. B., Hadiaty, R. K., de Alwis Goonatilake, S., Fernado, M. & Kotagama, O. (2019). Anabas testudineus (errata version published in 2020). The IUCN Red List of Threatened Species 2019: e.T166543A174787197.
Ahmadi, A., Lilimantik, E., & Sri Mahreda, E. (2021). Marketing Efficiency of the Climbing Perch (Anabas testudineus) Cultured with Bioflock System. Egyptian Journal of Aquatic Biology and Fisheries, 25(2), 561-572.
Amornsakun, T., Sriwatana, W., & Promkaew P. (2005). Some aspects in the early life stage of climbing perch, Anabas testudineus larvae. Songklanakarin Journal of Science and Technology, 27.
Arezo, M. J., D'Alessandro, S., Papa, N., de Sá, R., & Berois, N. (2007). Sex differentiation pattern in the annual fish Austrolebias charrua (Cyprinodontiformes: Rivulidae). Tissue & cell, 39(2), 89–98.
Baroiller, J. F., D'Cotta, H., & Saillant, E. (2009). Environmental effects on fish sex determination and differentiation. Sexual development: genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation, 3(2–3), 118–135.
Behera, S., Devi, L. M., Kumar, S., Gogoi, R., Samanta, P., Jomang, O., & Baksi, S. (2015). External morphology and sexual dimorphism of Anabas testudineus in natural environment. International Journal of Science and Nature, 6(2), 288–292.
Bhatta, S., Iwai, T., Miura, C., Higuchi, M., Shimizu-Yamaguchi, S., Fukada, H., & Miura, T. (2012a). Gonads directly regulate growth in teleosts. Proceedings of the National Academy of Sciences of the United States of America, 109(28), 11408–11412.
Bhatta, S., Iwai, T., Miura, T., Higuchi, M., Maugars, G., & Miura, C. (2012b). Differences between male and female growth and sexual maturation in tilapia (Oreochromis mossambicus). Kathmandu University Journal of Science, Engineering and Technology, 8, 57–65.
Bhuyan, S., & Hussain, S. (2018). Breeding and Seed Rearing of Climbing Perch (Anabas testudineus, Bloch) Using Farmer Friendly Innovative Technology at Farmer's Field: A Case Study. International Journal of Bioresource Science, 5, 101–105.
Brown, M. S., Evans, B. S., & Afonso, L. O. B. (2022). Developmental changes in gene expression and gonad morphology during sex differentiation in Atlantic salmon (Salmo salar). Gene, 823, 146393.
Chakraborty, S. B., & Banerjee, S. (2010). Comparative growth performance of mixed-sex and mono-sex Nile tilapia population in freshwater cage culture system under Indian perspective. International Journal of Biology, 2, 44–50.
Cho, H. C., Hwang, I. J., & Baek, H. J. (2014). Histological Analysis of Early Gonadal Development and Sex Differentiation in Chameleon Goby, Tridentiger trigonocephalus. Development & Reproduction, 18(1), 51–56.
Dan, N.C., & Little, D.C. (2000). The culture performance of mono-sex and mixed-sex new-season and overwintered fry in three strains of Nile tilapia (Oreochromis niloticus) in northern Vietnam. Aquaculture, 184, 221–231.
Fujimoto, T., Nishimura, T., Goto-Kazeto, R., Kawakami, Y., Yamaha, E., & Arai, K. (2010). Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proceedings of the National Academy of Sciences, 107(40), 17211–17216.
Genten, F., Terwinghe, E., & Danguy, A. (2009). Atlas of Fish Histology (1st ed.). Enfield (NH): Science Publishers.
Guiguen, Y., Fostier, A., Piferrer, F., & Chang, C. F. (2010). Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. General and Comparative Endocrinology, 165(3), 352–366.
Heule, C., Salzburger, W., & Böhne, A. (2014). Genetics of sexual development: an evolutionary playground for fish. Genetics, 196(3), 579–591.
Hidayat, R., Carman, O., & Alimuddin. (2016). Sexual dimorphism related to growth in climbing perch Anabas testudineus. Indonesian Journal of Aquaculture, 15(1), 8–14.
Hidayatulloh, D. R., Dhamayanti, Y., & Purnama, M. T. E. (2021). Morphometric and histological studies of the gonads of male and female hatchlings of olive ridley turtles (Lepidochelys olivacea). In IOP Conference Series: Earth and Environmental Science, 718(1), 012040.
Ijiri, S., Kaneko, H., Kobayashi, T., Wang, D. S., Sakai, F., Paul-Prasanth, B., Nakamura, M., & Nagahama, Y. (2008). Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biology of reproduction, 78(2), 333–341.
Jesus, L. W., Chehade, C., Costa, F. G., & Borella, M. I. (2013). Pituitary gland morphogenesis and ontogeny of adenohypophyseal cells of Salminus brasiliensis (Teleostei, Characiformes). Fish Physiology and Biochemistry, 40, 897–909.
Jiang, M., Jia, S., Chen, J., Chen, K., Ma, W., Wu, X., Luo, H., Li, Y., Zhu, Z., Hu, W. (2020). Timing of gonadal development and dimorphic expression of sex-related genes in gonads during early sex differentiation in the Yellow River carp. Aquaculture, 518(1), 734825.
Jí¸rgensen, A., Morthorst, J.E., & Andersen, O. (2008). Expression profiles for six zebrafish genes during gonadal sex differentiation. Reprod Biol Endocrinol 6, 25.
Kobayashi T. (2010). In vitro germ cell differentiation during sex differentiation in a teleost fish. The International journal of developmental biology, 54(1), 105–111.
Kobayashi, T., Kajiura-Kobayashi, H., Guan, G., & Nagahama, Y. (2008). Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus). Developmental Dynamics: an official publication of the American Association of Anatomists, 237(1), 297–306.
Kocour, M., Linhart, O., Gela, D., & Rodina, M. (2005). Growth performance of all"female and mixed"sex common carp Cyprinus carpio L. populations in the Central Europe climatic conditions. Journal of the World Aquaculture Society, 36(1), 103–113.
Maitre, D., Selmoni, O., Uppal, A., Marques da Cunha, L., Wilkins, L., Roux, J., Mobley, K., Castro, I., Knörr, S., Robinson-Rechavi, M., Wedekind, C. (2017). Sex differentiation in grayling (Salmonidae) goes through an all-male stage and is delayed in genetic males who instead grow faster. Scientific Reports, 7.
Mazzoni, T. S., & Grassiotto, I. Q. (2017). Ovary Differentiation and Activity in Teleostei Fish. InTech.
Mondal, M. N., Shahin, J., Wahab, M. A., Asaduzzaman, M., & Yang, Y. (2010). Comparison between cage and pond production of Thai Climbing Perch (Anabas testudineus) and Tilapia (Oreochromis niloticus) under three management systems. Journal of the Bangladesh Agricultural University, 8, 313–322.
Mondal, Md. Nurunnabi & Shahin, J. & Wahab, Md & Asaduzzaman, Md & Yang, Y.. (2010). Comparison between cage and pond production of Thai Climbing Perch (Anabas testudineus) and Tilapia (Oreochromis niloticus) under three management systems. Bangladesh Journal of Agricultural Research, 8.
Morioka, S., Ito, S., Kitamura, S., Vongvichith, B. (2009). Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus. Ichthyological Research, 56, 162–171.
Murata, R., Karimata, H., Kobayashi, Y., Horiguchi, R., Kishimoto, K., Kimura, M., Kobayashi, T., Soyano, K., & Nakamura, M. (2011). Differentiation of steroid-producing cells during ovarian differentiation in the protogynous Malabar grouper, Epinephelus malabaricus. The International journal of developmental biology, 55(6), 619–625.
Nagahama Y. (2005). Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish physiology and biochemistry, 31(2–3), 105–109.
Nakamura, M. (2013). Morphological and Physiological Studies on Gonadal Sex Differentiation in Teleost Fish. Aqua-bioscience Monographs, 6, 1–47.
Nakamura, M., Bhandari, R. K., & Higa, M. (2003). The role estrogens play in sex differentiation and sex changes of fish. Fish Physiology and Biochemistry, 28, 113–117.
Nargis, A. (2010). Aging and Growth Records of Anabas testudineus (Bloch) (Anabantidae: Perciformes). Bangladesh Journal of Scientific and Industrial Research, 45, 283–287.
Okutsu, T., Suzuki, K., Takeuchi, Y., Takeuchi, T., & Yoshizaki, G. (2006). Testicular germ cells can colonize sexually undifferentiated embryonic gonads and produce functional eggs in fish. Proceedings of the National Academy of Sciences of the United States of America, 103(8), 2725–2729.
Pan, Z. J., Zhu, C. K., Wang, H., Zhou, F. J. & Qiang, X. G. (2017), Gonadal morphogenesis and sex differentiation in cultured Ussuri catfish Tachysurus ussuriensis. Journal Fish Biology, 91, 866–879.
Pandian, T. J. (2013). Endocrine Sex Differentiation in Fish (1st ed.). Boca Raton: CRC Press.
Penman, D. J., & Piferrer, F. (2008). Fish gonadogenesis. Part I: genetic and environmental mechanisms of sex determination. Reviews in Fisheries Science, 16(sup1), 16–34.
Piferrer, F. (2001). Endocrine sex control strategies for the feminization of teleost fish. Aquaculture, 197(1–4), 229–281.
Piferrer, F. (2011). Hormonal Control of Reproduction and Growth: Endocrine control of sex differentiation in fish. In Farrell AP (Ed), Encyclopedia of Fish Physiology, From Genome to Environment. (pp. 1490–1499). San Diego: Academic Press.
Piferrer, F., & Guiguen, Y. (2008). Fish Gonadogenesis. Part II: Molecular Biology and Genomics of Sex Differentiation. Reviews in Fisheries Science, 16, 35–55.
Rahmadi, R., Syahril, A., Nur, F. M., Maulida, S., & Muchlisin, Z. A. (2021). Embryogenesis of climbing perch fish Anabas testudineus Bloch 1792 at an incubation temperature of 28°C. IOP Conference Series. Earth and Environmental Science, 869 (1).
Sakai, F., Kobayashi, T., Matsuda, M., & Nagahama, Y. (2008). Stability in aromatase immunoreactivity of steroid-producing cells during early development of XX gonads of the Nile tilapia, Oreochromis niloticus: an organ culture study. Zoological science, 25(3), 344–348.
Sarker, M. A., Arshad, F. M., Alam, M. F., Mohamed, Z. A., & Khan, M. A. (2014). Economics of Thai koi (climbing perch, Anabas testudineus) farming in a pond in Bangladesh. Trends in Agricultural Economics, 7(1), 26–40.
Siegfried, K. R., & Nüsslein-Volhard, C. (2008). Germline control of female sex determination in zebrafish. Developmental biology, 324(2), 277–287.
Tong, S. K., Hsu, H. J., & Chung, B. C. (2010). Zebrafish mono-sex population reveals female dominance in sex determination and earliest events of gonad differentiation. Developmental biology, 344(2), 849–856.
Uddin, K. B., Moniruzzaman, M., Bashar, M. A., Basak, S., Islam, A. S., Mahmud, Y., & Bai, S. C. (2016). Culture potential of Thai climbing perch (Anabas testudineus) in experimental cages at different stocking densities in Kaptai Lake, Bangladesh. Aquaculture, Aquarium, Conservation & Legislation, 9(3), 564–573.
Vidal-López, J. M., Contreras-Sánchez, W. M., Torres"Martínez, A., Hernández-Franyutti, A. A., & Uribe, M. C. (2018). Early gonadal differentiation in the Mexican snook Centropomus poeyi (Centropomidae, Perciformes, Teleostei) suggests protandric hermaphroditism. Marine and Freshwater Behaviour and Physiology, 51, 327–345.
Vizziano, D., Randuineau, G., Baron, D., Cauty, C., & Guiguen, Y. (2007). Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss. Developmental dynamics: an official publication of the American Association of Anatomists, 236(8), 2198–2206.
von Hofsten, J., & Olsson, P. E. (2005). Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reproductive biology and endocrinology: RB&E, 3, 63.
Ye, D., Zhu, L., Zhang, Q., Xiong, F., Wang, H., Wang, X., He, M., Zhu, Z., & Sun, Y. (2019). Abundance of Early Embryonic Primordial Germ Cells Promotes Zebrafish Female Differentiation as Revealed by Lifetime Labeling of Germline. Marine biotechnology (New York, N.Y.), 21(2), 217–228.
Yön, N. D., & Akbulut, C. (2015). Identification of primordial germ cells: cytological, histological and immunohistochemical aspects. Brazilian Archives of Biology and Technology, 58, 222–228.
Zhang, J., Huang, J. Q., Li, Y. Y., Wang, N., Sun, J., Wang, T. Z., & Chen, S. L. (2013). Histological observations of Medaka (Oryzias latipes) gonad sexual differentiation and development. Zoological Research, 34(5), 464–470.
Zhang, Q., Sun, X., Qi, J., Wang, Z., Wang, X., Wang, X., & Zhai, T. (2009). Sex determination mechanisms in fish. Journal of Ocean University of China, 8, 155–160.
Copyright (c) 2024 Rahmat Hidayat, Odang Carman, Alimuddin Alimuddin

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish in this journal agree to the following terms:
1. The journal allows the author to hold the copyright of the article without restrictions;
2. The journal allows the author(s) to retain publishing rights without restrictions;
3. The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA).






11.jpg)