Vector Surveillance for Lymphatic Filariasis After Mass Drug Administration in an Endemic Area: A Case Study in Bekasi
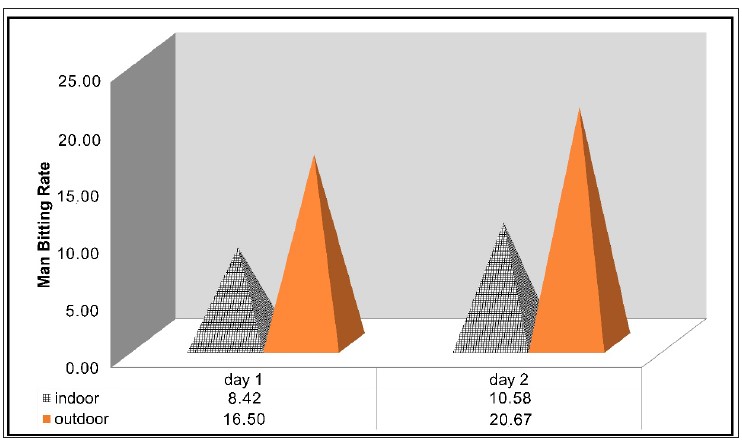
Introduction: Lymphatic Filariasis (LF) re-transmission in endemic areas that have completed mass drug administration (MDA) should be a concern. Entomological data are required to support the elimination of LF. This research aims to present bionomic and evaluative evidence of Wuchereria bancrofti in Culex quinquefasciatus in Bekasi. Methods: Entomological surveys were carried out in Jatimulya Village, Bekasi, from October to November 2019. Female Cx. quinquefasciatus were caught using Human-baited Double Net traps (HDNs) both indoors and outdoors over a 12-hours (from 6 PM to 6 AM). Female mosquitos were subjected to ovary dissection to determine their longevity. In addition, the Man-Hour Density (MHD), Man-Biting Rate (MBR), Daily Survival Rate (DSR), and estimated longevity were calculated. Wuchereria bancrofti was detected using the Polymerase Chain Reaction (PCR) on dissected mosquitos. Results and Discussion: In total 673 female Cx. quinquefasciatus were collected. Culex quinquefasciatus' peak landing time was demonstrated between 12 and 3 AM. The values of Mosquito Parity Rate (MPR) and DSR are 22.88 and 0.692, respectively, implying that the estimated lifespan of dissected mosquitos ranged up to three days. The PCR analysis has revealed that none of the 48 pooled samples of Cx. quinquefasciatus are tested positive for W. bancrofti. Conclusion: Although this survey has found non-existent microfilaria in the LF vector Cx. quinquefasciatus, routine vector monitoring, and surveillance are still required to ensure the long-term viability of the LF elimination program.
van ‘t Noordende AT, Aycheh MW, Schippers A. The Impact of Leprosy, Podoconiosis and Lymphatic Filariasis on Family Quality of Life: A Qualitative Study in Northwest Ethiopia. PLoS Negl Trop Dis. 2020;14(3):e0008173. https://doi.org/10.1371/journal.pntd.0008173
Kebede B, Martindale S, Mengistu B, Kebede B, Mengiste A, H/Kiros F, et al. Integrated Morbidity Mapping of Lymphatic Filariasis and Podoconiosis Cases in 20 Co-Endemic Districts of Ethiopia. PLoS Negl Trop Dis. 2018;12(7):e0006491. https://doi.org/10.1371/journal.pntd.0006491
Ali O, Deribe K, Semrau M, Mengiste A, Kinfe M, Tesfaye A, et al. A Cross-Sectional Studyto Evaluate Depression and Quality of Life Among Patients with Lymphoedema Due to Podoconiosis, Lymphatic Filariasis and Leprosy. Trans R Soc Trop Med Hyg. 2020;114(12):983–994. https://doi.org/10.1093/trstmh/traa130
Reasoa MS, Ranimpi YY, Kurniasari RRMD, De Fretes F. Respon Psikososial dan Kesejahteraan Psikologis Pasien Filariasis di Kota Ambon. J Psikol Ulayat. 2020;7(1):24–37. https://doi.org/10.24854/Jpu02019-230
World Health Organization. Lymphatic Filariasis. Geneva: World Health Organization; 2022. https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis
Deshpande A, Miller-Petrie MK, Johnson KB, Abdoli A, Abrigo MRM, Adekanmbi V, et al. The Global Distribution of Lymphatic Filariasis, 2000–18: A Geospatial Analysis. Lancet Glob Heal. 2020;8(9):e1186–e1194. https://doi.org/10.1016/S2214-109x(20)30286-2
Lee J, Ryu JS. Current Status of Parasite Infections In Indonesia: A Literature Review. Korean J Parasitol. 2019;57(4):329–339. https://doi.org/10.3347/Kjp.2019.57.4.329
World Health Organization. Global Programme to Eliminate Lymphatic Filariasis: Progress Report, 2021. Wkly Epidemiol Rec. 2022;97(14):513–524. https://www.who.int/publications/i/item/who-wer9641-497-508
Rebollo MP, Bockarie MJ. Can Lymphatic Filariasis Be Eliminated by 2020?. Trends Parasitol. 2017;33(2):83–92. https://doi.org/10.1016/J.Pt.2016.09.009
Directorate of Vector-Infected and Zoonotic Diseases. Action Plans of Vector and Zoonotic Disease Prevention and Control Activities in 2015-2019. Jakarta: Ministry of Health of Republic Indonesia. 2017. https://e-renggar.kemkes.go.id/file2018/e-performance/1-465842-4tahunan-265.pdf
Ministry of Health of Republic Indonesia. Indonesia Health Profile 2021. Jakarta: Ministry of Health of Republic Indonesia; 2022. 1–289 p. https://www.kemkes.go.id/downloads/resources/download/pusdatin/profil-kesehatan-indonesia/Profil-Kesehatan-2021.pdf
Ministry of Health of Republic Indonesia. Indonesia Health Profile 2019. Jakarta: Ministry of Health of Republic Indonesia; 2020. 1–256 p. https://www.kemkes.go.id/downloads/resources/download/pusdatin/profil-kesehatan-indonesia/Profil-Kesehatan-Indonesia-2019.pdf
World Health Organization. Monitoring and Epidemiological Assessment of Mass Drug Administration; Global Programme to Eliminate Lymphatic : a Manual for National Elimination Programmes. Geneva: WHO Press; 2011. 1–78 p. https://apps.who.int/iris/bitstream/handle/10665/44580/9789241501484_eng.pdf?sequence=1&isAllowed=y
Goldberg EM, King JD, Mupfasoni D, Kwong K, Hay SI, Pigott DM, et al. Ecological and Socioeconomic Predictors of Transmission Assessment Survey Failure for Lymphatic Filariasis. Am J Trop Med Hyg. 2019;101(1):271–278. https://doi.org/10.4269/Ajtmh.18-0721
World Health Organization. Lymphatic Filariasis: Monitoring and Epidemiological Assessment of Mass Drug Administration. Ichimori K, editor. World Health Organization Global Programme to Eliminate Lymphatic Filariasis. Geneva, Switzerland: WHO Press; 2011. https://apps.who.int/iris/handle/10665/44580
Santoso, Yahya, Supranelfy Y, Suryaningtyas NH, Taviv Y, Yenni A, et al. Risk of Recrudescence of Lymphatic Filariasis after Post-MDA Surveillance in Brugia Malayi Endemic Belitung District, Indonesia. Korean J Parasitol. 2020;58(6):627–634. https://doi.org/10.3347/Kjp.2020.58.6.627
Subramanian S, Jambulingam P, Krishnamoorthy K, Sivagnaname N, Sadanandane C, Vasuki V, et al. Molecular Xenomonitoring As A Post-MDA Surveillance Tool for Global Programme to Eliminate Lymphatic Filariasis: Field Validation in an Evaluation Unit in India. PLoS Negl Trop Dis. 2020;14(1):1–25. https://doi.org/10.1371/Journal.Pntd.0007862
McPherson B, Mayfield HJ, McLure A, Gass K, Naseri T, Thomsen R, et al. Evaluating Molecular Xenomonitoring as a Tool for Lymphatic Filariasis Surveillance in Samoa, 2018–2019. Trop Med Infect Dis. 2022;7(8):203. https://doi.org/10.3390/Tropicalmed7080203
Irish SR, Al-Amin HM, Paulin HN, Mahmood ASMS, Khan RK, Muraduzzaman AKM, et al. Molecular Xenomonitoring for Wuchereria Bancrofti in Culex Quinquefasciatus in Two Districts in Bangladesh Supports Transmission Assessment Survey Findings. PLoS Negl Trop Dis. 2018;12(7):e0006574. https://doi.org/10.1371/Journal.Pntd.0006574
Subramanian S, Jambulingam P, Chu BK, Sadanandane C, Vasuki V, Srividya A, et al. Application of A Household-Based Molecular Xenomonitoring Strategy to Evaluate The Lymphatic Filariasis Elimination Program In Tamil Nadu, India. PLoS Negl Trop Dis. 2017;11(4):e0005519. https://doi.org/10.1371/Journal.Pntd.0005519
Rao RU, Samarasekera SD, Nagodavithana KC, Dassanayaka TDM, Punchihewa MW, Ranasinghe USB, et al. Reassessment of Areas with Persistent Lymphatic Filariasis Nine Years After Cessation of Mass Drug Administration In Sri Lanka. PLoS Negl Trop Dis. 2017;11(10):e0006066. https://doi.org/10.1371/Journal.Pntd.0006066
Moustafa MA, Salamah MMI, Thabet HS, Tawfik RA, Mehrez MM, Hamdy DM. Molecular Xenomonitoring (MX) And Transmission Assessment Survey (TAS) of Lymphatic Filariasis Elimination in Two Villages, Menoufyia Governorate. Egypt. Eur J Clin Microbiol Infect Dis. 2017;36(7):1143–1150. https://doi.org/10.1007/S10096-017-2901-3
Ramesh A, Cameron M, Spence K, Hoek Spaans R, Melo-Santos MA V, Paiva MHS, et al. Development of an Urban Molecular Xenomonitoring System for Lymphatic Filariasis in the Recife Metropolitan Region, Brazil. PLoS Negl Trop Dis. 2018;12(10):e0006816. https://doi.org/10.1371/Journal.Pntd.0006816
Dorkenoo MA, De Souza DK, Apetogbo Y, Oboussoumi K, Yehadji D, Tchalim M, et al. Molecular Xenomonitoring for Post-Validation Surveillance of Lymphatic Filariasis in Togo: No Evidence for Active Transmission. Parasites and Vectors. 2018;11(52):1-9. https://doi.org/10.1186/S13071-017-2611-9
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, Richardson JH. Illustrated Keys to the Mosquitoes of Thailand. Southeast Asian J Trop Med Public Health. 2010;41 Suppl 1(2):1–225. http://www.ncbi.nlm.nih.gov/pubmed/20629439
World Health Organization. Manual on Practical Entomology in Malaria. Geneva: World Health Organization;1975. https://apps.who.int/iris/handle/10665/42481
Saeed M, Siddiqui S, Bajpai P, K.Srivastava A, Mustafa H. Amplification of Brugia Malayi DNA Using Hha1 Primer as A Tool. Open Conf Proc J. 2015;5(1):38–40. https://doi.org/10.2174/2210289201405030038
Warrell DA, Gilles HM. Essential Malariology. Oxford University Press Inc.; 2002. 348 p.
Setiyaningsih R, Anggraeni YM, Mujiyono, Yanti AO, Mujiyanto, Garjito TA, et al. Bio-Ecological Study of Culex Quinquefasciatus as a Potential Vector of Japanese Encephalitis In Some Provinces In Indonesia. IOP Conf Ser Earth Environ Sci. 2021;948(1):012036. https://doi.org/10.1088/1755-1315/948/1/012036
Portunasari WD, Kusmintarsih ES, Riwidiharso E. Survei Nyamuk Culex spp. sebagai Vektor Filariasis di Desa Cisayong, Kecamatan Cisayong, Kabupaten Tasikmalaya. Biosfera. 2017;33(3):142–148. https://doi.org/10.20884/1.mib.2016.33.3.361
Astuti EP, Widawati M, Yuliasih Y, Ruliansyah A, Kusnandar AJ. Lama Hidup dan Potensi Culex Quinquefasciatus sebagai Vektor Filariasis Limfatik Berdasarkan Ketinggian Pasca Transmission Assesment Survey (TAS) di Kabupaten Subang, Jawa Barat. Vektora J Vektor dan Reserv Penyakit. 2020;12(2):155–166. https://doi.org/10.22435/Vk.V12i2.3241
Nirwan M, Hadi UK, Soviana S, Satrija F, Setiyaningsih S. Diversity, Domination and Behavior of Mosquitoes in Filariasis Endemic Area of Bogor District, West Java, Indonesia. Biodiversitas. 2022;23(4):2093–2100. https://doi.org/10.13057/Biodiv/D230444
Prasetyowati H, Riandi MU, Hendri J, Ipa M. Entomological Assessment in Tangerang, Indonesia: Post Transmission Assessment Survey of Lymphatic Filariasis Endemic Villages. In: Proceedings of the 5th Universitas Ahmad Dahlan Public Health Conference (UPHEC 2019). 2020;1(1): 67–71. https://doi.org/10.2991/ahsr.k.200311.012
Directorate General of Disease Control and Environmental Health. Epidemiology of Elephantiasis (Filariasis) in Indonesia. Jakarta: Health Department of Republic Indonesia; 2008.
Sukendra DM, Santik YDP, Wahyono BW, Siyam N, Indrawati F. The Influence of Vegetation and House Index on Male Mosquitoes DHF Vector Abundance on Kawengen Sub-District. Unnes J Public Heal. 2020;9(1):64–70. https://doi.org/10.15294/ujph.v9i1.34714
Dickson BFR, Graves PM, McBride WJ. Lymphatic Filariasis In Mainland Southeast Asia: A Systematic Review and Meta-Analysis of Prevalence and Disease Burden. Trop Med Infect Dis. 2017;2(3):32. https://doi.org/10.3390/Tropicalmed2030032
Hoedojo. Vector of Malaria and Filariasis in Indonesia. Bul Penelit Kesehat. 1989;17(2):180–190. http://ejournal.litbang.kemkes.go.id/index.php/BPK/article/view/658
Nasution SFI, Adhiyanto C, Indahwati E. Preliminary Study of Wuchereria bancrofti L3 Larvae Detection in Culex quinquefasciatus as Vector Potential of Filariasis in Endemic Area of South Tangerang, By Utilizing Assay for L3-Activated Cuticlin Transcript mRNA Gene and TPH-1 Gene. Indones J Trop Infect Dis. 2018;7(3):67–72. https://doi.org/10.20473/ijtid.v7i3.7352
Ramadhani T, Sumarni S. Culex Quinquifasciatus as the Main Vector of Lymphatic Filariasis Caused by Wuchereria Bancrofti in Pabean Village Pekalongan City. J Ekol Kesehat. 2010;9(3):1303–1310. https://ejournal.litbang.kemkes.go.id/index.php/jek/article/view/5386
Nurjazuli. Entomology Survey Based on Lymphatic Filariasis Locus in the District of Pekalongan City Indonesia. Int J Sci Basic Appl Res. 2015;22(1):295–302. https://gssrr.org/index.php/JournalOfBasicAndApplied/article/view/3933
Juhairiyah J, Hidayat S, Hairani B, Fakhrizal D, Setyaningtyas DE. Keanekaragaman Jenis dan Perilaku Nyamuk pada Daerah Endemis Filariasis di Kabupaten Barito Kuala, Provinsi Kalimantan Selatan. BALABA. 2018;14(1):31–42. https://doi.org/10.22435/Blb.V14i1.296
Ramadhani T, Wahyudi BF. Keanekaragaman dan Dominasi Nyamuk di Daerah Endemis Filariasis Limfatik, Kota Pekalongan. J Vektor Penyakit. 2016;9(1):1–8. https://doi.org/10.22435/Vektorp.V9i1.5037.1-8
Rangkuti AF, Sulistyani S, Endah W N. Faktor Lingkungan dan Perilaku yang Berhubungan dengan Kejadian Malaria di Kecamatan Panyabungan Mandailing Natal Sumatera Utara. BALABA. 2018;13(1):1–10. https://doi.org/10.22435/blb.v13i1.238
Cardo MV, Rubio A, Junges MT, Vezzani D, Carbajo AE. Heterogeneous Distribution of Culex Pipiens, Culex Quinquefasciatus and Their Hybrids Along the Urbanisation Gradient. Acta Trop. 2018;178(1):229–235. https://doi.org/10.1016/J.Actatropica.2017.11.017
Alkhayat FA, Ahmad AH, Rahim J, Dieng H, Ismail BA, Imran M, et al. Charaterization of Mosquito Larval Habitats in Qatar. Saudi J Biol Sci. 2020;27(9):2358–2365. https://doi.org/10.1016/J.Sjbs.2020.07.006
Dida GO, Anyona DN, Abuom PO, Akoko D, Adoka SO, Matano AS, et al. Spatial Distribution and Habitat Characterization of Mosquito Species During the Dry Season Along the Mara River and Its Tributaries, in Kenya and Tanzania. Infect Dis Poverty. 2018;7(2):1-16. https://doi.org/10.1186/S40249-017-0385-0
McClure KM, Lawrence C, Kilpatrick AM. Land Use and Larval Habitat Increase Aedes Albopictus (Diptera: Culicidae) and Culex Quinquefasciatus (Diptera: Culicidae) Abundance In Lowland Hawaii. J Med Entomol. 2018;55(6):1509–1516. https://doi.org/10.1093/Jme/Tjy117
Asigau S, Parker PG. The Influence of Ecological Factors on Mosquito Abundance and Occurrence in Galápagos. J Vector Ecol. 2018;43(1):125–137. https://doi.org/10.1111/Jvec.12292
Rahman MA, Yahathugoda TC, Tojo B, Premaratne P, Nagaoka F, Takagi H, et al. A Surveillance System for Lymphatic Filariasis After Its Elimination in Sri Lanka. Parasitol Int. 2019;68(1):73–78. https://doi.org/10.1016/J.Parint.2018.10.003
García-Rejón JE, Farfan-Ale JA, Ulloa A, Flores-Flores LF, Rosado-Paredes E, Baak-Baak C, et al. Gonotrophic Cycle Estimate for Culex quinquefasciatus in Mérida, Yucatán, México. J Am Mosq Control Assoc. 2008;24(3):344–348. https://doi.org/10.2987/5667.1

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of all journal manuscripts is held by the Jurnal Kesehatan Lingkungan.2. Formal legal provisions to access digital articles of electronic journal are subject to the provision of the Creative Commons Attribution-ShareAlike license (CC BY-NC-SA), which means that Jurnal Kesehatan Lingkungan is rightful to keep, transfer media/format, manage in the form of databases, maintain, and publish articles.
3. Published manuscripts both printed and electronic are open access for educational, research, and library purposes. Additionally, the editorial board is not responsible for any violations of copyright law.
JKESLING by UNAIR is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.