Adsorption Kinetics of Banana Stem Activated Carbon in Reducing Phosphate Levels
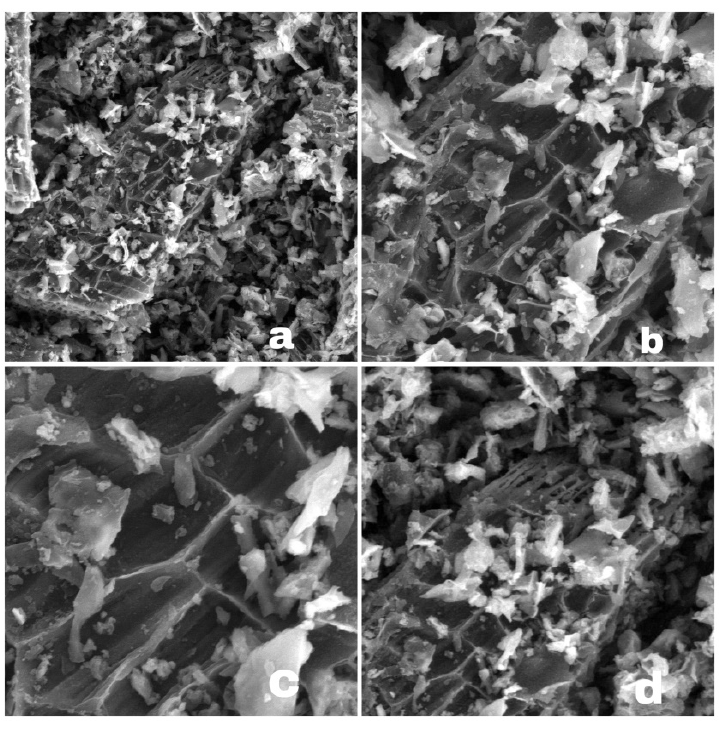
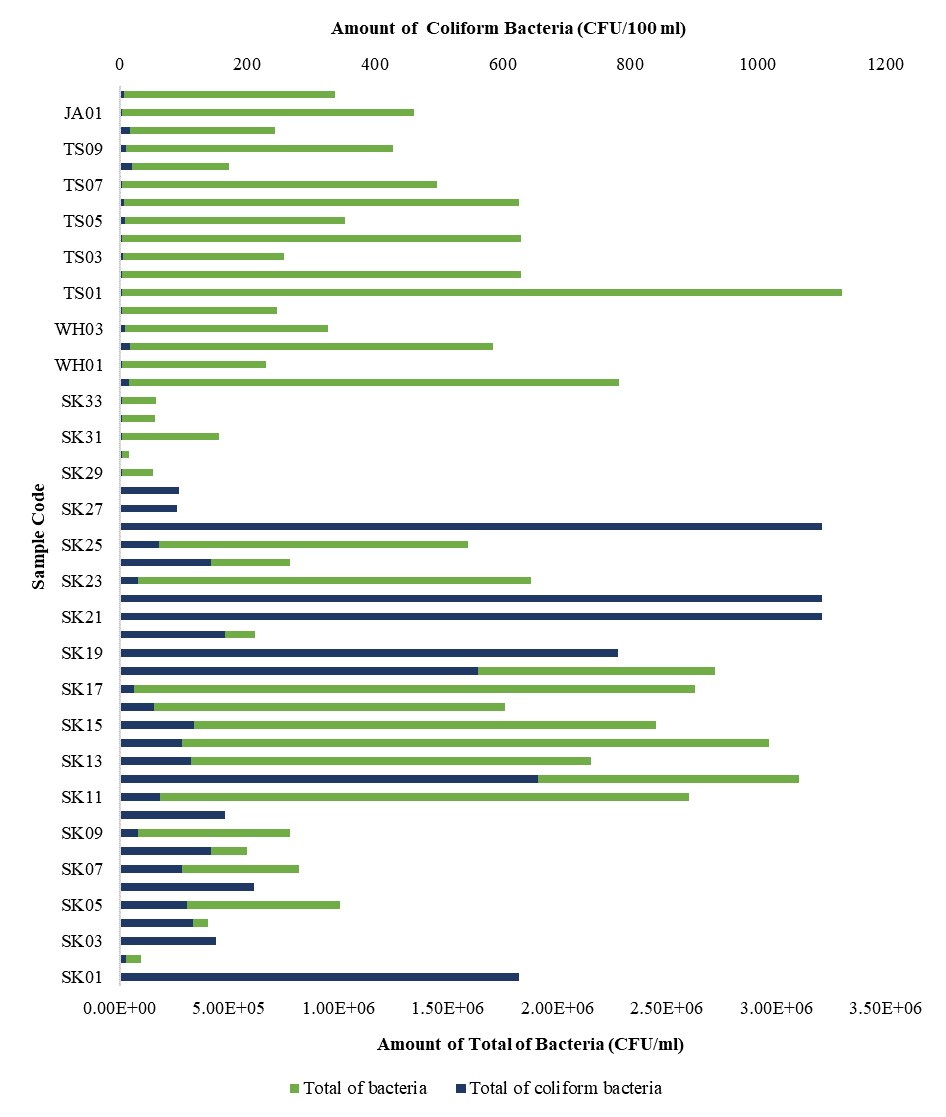
Introduction: High levels of phosphate in water are caused by wastewater pollution such as laundry waste water causing eutrophication. Adsorption is a method that can be used to reduce phosphate level. Banana stem that contains high levels of cellulose can be used as the main ingredient for making activated carbon. The aims of this study were to analyze the adsorption capacity and adsorption kinetics of banana stem activateld carbon in relducing phosphatel lelvells. Methods: The design of this research was true experiment with a pretest-posttest controlled group. Adsorption process was carried out with batch method that used three variations of adsorbate concentration and four variations of mixing time. Adsorption took place at pH 3 and 30 rpm of mixing time. Adsorption capacity was analyzed using the Langmuir isotherm and Freundlich isotherm models. Adsorption kinetic was analyzed using pseudo-first-order and pseudo-second-order kinetic models. Results and Discussion: The X-Ray Diffraction (XRD) difactogram and Scanning Electron Microscopy (SEM) results prove that the activateld carbon was successfully made. The iodine number of banana stelm activateld carbon was 698.12 mg/g. The results showed that activated carbon from banana stem successfully reduced phosphate levels in water with adsorption capacity 0.10708 mg/g and following the Langmuir isotherm model. The kinetics adsorption of banana stem activated carbon was validated by the pseludo-selcond-ordelr kinetics model with a kinetics constant of 0.17137 g/mg.min. Conclusion: The Langmuir models indicated that adsorption of phosphate occurred in monolayer. Modifications of activated carbon were needed based on characterization results.
Almanassra IW, Mckay G, Kochkodan V, Ali Atieh M, Al-Ansari T. A Atate of the Art Review on Phosphate Removal from Water by Biochars. Chem Eng J. 2021;409(128211):1-15. https://doi.org/10.1016/j.cej.2020.128211
Utomo WP, Nugraheni ZV, Rosyidah A, Shafwah OM, Naashihah LK, Nurfitria N, et al. Penurunan Kadar Surfaktan Anionik dan Fosfat dalam Air Limbah Laundry di Kawasan Keputih, Surabaya menggunakan Karbon Aktif. Akta Kim Indones. 2018;3(1):127-140. https://doi.org/10.12962/j25493736.v3i1.3528
Isiuku BO, Enyoh CE. Pollution and Health Risks Assessment of Nitrate and Phosphate Concentrations in Water Bodies in South Eastern, Nigeria. Environ Adv. 2020;2(100018):1-8. https://doi.org/10.1016/j.envadv.2020.100018
Kumwimba MN, Dzakpasu M, Li X. Potential of Invasive Watermilfoil (Myriophyllum spp.) to Remediate Eutrophic Waterbodies with Organic and Inorganic Pollutants. J Environ Manage. 2020;270(110919):1-17. https://doi.org/10.1016/j.jenvman.2020.110919
Mustikarani F, Saziati O. Efisiensi Pengolahan Fosfat Limbah Cair Laundry Menggunakan Karbon Aktif Sampah Plastik PVC dan LDPE. Beranda. 2022;10(3):222–231. https://doi.org/10.26760/rekalingkungan.v10i3.222-231
Almanassra IW, Kochkodan V, Mckay G, Atieh MA, Al-Ansari T. Review of Phosphate Removal from Water by Carbonaceous Sorbents. J Environ Manage. 2021;287(112245):1-17. https://doi.org/10.1016/j.jenvman.2021.112245
Karim MA, Juniar H, Ambarsari MFP. Adsorpsi Ion Logam Fe Dalam Limbah Tekstil Sintesis dengan Menggunakan Metode Batch. J Distilasi. 2018;2(2):68-81. https://doi.org/10.32502/jd.v2i2.1205
Anggriani UM, Hasan A, Purnamasari I. Kinetika Adsorpsi Karbon Aktif dalam Penurunan Konsentrasi Logam Tembaga (Cu) dan Timbal (Pb). Univ Sriwij. 2021;12(02):29–37. https://jurnal.polsri.ac.id/index.php/kimia/index
Setyorini D, Arninda A, Syafaatullah AQ, Panjaitan R. Penentuan Konstanta Isoterm Freundlich dan Kinetika Adsorpsi Karbon Aktif Terhadap Asam Asetat. Eksergi. 2023;20(3):149-155. https://doi.org/10.31315/e.v20i3.10835
Musah M, Azeh Y, Mathew J, Umar M, Abdulhamid Z, Muhammad A. Adsorption Kinetics and Isotherm Models: A Review. Caliphate J Sci Technol. 2022;4(1):20–26. https://doi.org/10.4314/cajost.v4i1.3
Indonesian Central Bureau of Statistic. Produksi Pisang Menurut Provinsi, Tahun 2015-2019. Jakarta: Indonesian Central Bureau of Statistic; 2019.
Purwitasari DG, Tussania R, Fathoni R. Adsorpsi Logam Kadmium (Cd) pada Kadmium Sulfat (CdSO4) Menggunakan Batang Pohon Pisang Sebagai Adsorben. J Chemurg. 2022;6(1):131-136. https://doi.org/10.30872/cmg.v6i1.7905
Irwanto M, Sentosa A, Nugroho RA, Terbatas R. Pisang (Musa paradisiaca) sebagai Adsorben H2S di Ruang FTIR (Fourier Transform Infra - Red). J Rev Pend Peng. 2023;6(4):626–632. https://doi.org/10.31004/jrpp.v6i4.20201
Nuryoto N, Andini SD, Fauziah S, Filiandini E. Uji Coba Arang Batang Pisang Teraktivasi pada Pendegradasian Fosfat pada Limbah Laundry. J Inov Tek Kim. 2022;7(2):39-46. https://doi.org/10.31942/inteka.v7i2.6972
Wardani GA, Wulandari WT, Khoirunnisa R, Ningsih WR. Effect of Time Variation of Banana (Musa acuminate) leaf waste on lead metal adsorption. AIP Conf Proc. 2018;2049(030012):1-5 https://doi.org/10.1063/1.5082513
Raihan R, Setiawan A, Hakim L, Muhammad M, Arif M, Hosseiniamoli H. Preparation and Characterization of Activated Carbon Made from Robusta Coffee Skin (Coffea Canephora). J Rekayasa Kim Lingkung. 2020;15(2):104–110. https://doi.org/10.23955/rkl.v15i2.176
Miyazato T, Nuryono N, Kobune M, Rusdiarso B, Otomo R, Kamiya Y. Phosphate Recovery from an Aqueous Solution Through Adsorption-Desorption Cycle Over Thermally Treated Activated Carbon. J Water Process Eng. 2020;36(101302):1-8. https://doi.org/10.1016/j.jwpe.2020.101302
Yustinah, Hudzaifah, Aprilia M, Syamsudin AB. Kesetimbangan Adsorpsi Logam Berat (Pb) dengan Adsorben Tanah Diatomit Secara Batch. J Konversi. 2019;9(1):17-27. https://jurnal.umj.ac.id/index.php/konversi/article/view/7083
Sartika Z, Mariana M, Supardan MD. Penurunan Kadar COD, BOD dan Nitrit Limbah Pabrik Tahu Menggunakan Karbon Aktif Ampas Bubuk Kopi. J Serambi Eng. 2019;4(2):557–564. https://doi.org/10.32672/jse.v4i2.1334
Karthikeyan P, Meenakshi S. Synthesis and Characterization of Zn–Al LDHs/activated Carbon Composite and Its Adsorption Properties for Phosphate and Nitrate Ions in Aqueous Medium. J Mol Liq. 2019;296(111766):1-9. https://doi.org/10.1016/j.molliq.2019.111766
Nainggolan ITAB, Herman S, Reni Yenti S. Penentuan Model Kesetimbangan Adsorpsi Ion Fosfat Menggunakan Arang Aktif Tongkol Jagung dengan Variasi Massa dan Kecepatan Pengadukan. Jom FTEKNIK. 2019;6(1):1–8. https://jom.unri.ac.id/index.php/JOMFTEKNIK/article/view/24065/23295
Miri NSS, Narimo. Review : Kajian Persamaan Isoterm Langmuir dan Freundlich pada Adsorpsi Logam Berat Fe (II) dengan Zeolit dan Karbon Aktif dari Biomassa. J Kim dan Rekayasa. 2022;2(2):58–71. https://doi.org/10.31001/jkireka.v2i2.36d
Ragadhita R, Nandiyanto ABD. How to Calculate Adsorption Isotherms of Particles Using Two-Parameter Monolayer Adsorption Models and Equations. Indones J Sci Technol. 2021;6(1):205–234. https://doi.org/10.17509/ijost.v6i1.32354
Amelia H, Fitria R, Sunardi S. Kajian Isoterm Adsorpsi Metilen Biru pada Biochar Kulit Sagu (Metroxylon sagu). J Sains dan Teknol. 2023;6(1):135-142. https://journal.ummat.ac.id/index.php/justek/article/view/13746
Isiuku BO, Enyoh CE, Duru CE, Ibe FC. Current Research in Green and Sustainable Chemistry Phosphate Ions Removal from Aqueous Phase by Batch Adsorption on Activated (Activation Before Carbonization) Biochar Derived from Rubber Pod Husk. Environ Adv. 2021;4(100136):1-8. https://doi.org/10.1016/j.crgsc.2021.100136
Nazarian R, Desch RJ, Thiel SW. Kinetics and Equilibrium Adsorption of Phosphate on Lanthanum Oxide Supported on Activated Carbon. Colloids Surfaces A Physicochem Eng Asp. 2021;624(126813):1-11. https://doi.org/10.1016/j.colsurfa.2021.126813

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of all journal manuscripts is held by the Jurnal Kesehatan Lingkungan.2. Formal legal provisions to access digital articles of electronic journal are subject to the provision of the Creative Commons Attribution-ShareAlike license (CC BY-NC-SA), which means that Jurnal Kesehatan Lingkungan is rightful to keep, transfer media/format, manage in the form of databases, maintain, and publish articles.
3. Published manuscripts both printed and electronic are open access for educational, research, and library purposes. Additionally, the editorial board is not responsible for any violations of copyright law.
JKESLING by UNAIR is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.