Optimized RT-qPCR Detection of Hepatic Lipopolysaccharide-Binding Protein in Diet-Induced Obese Mice
Downloads
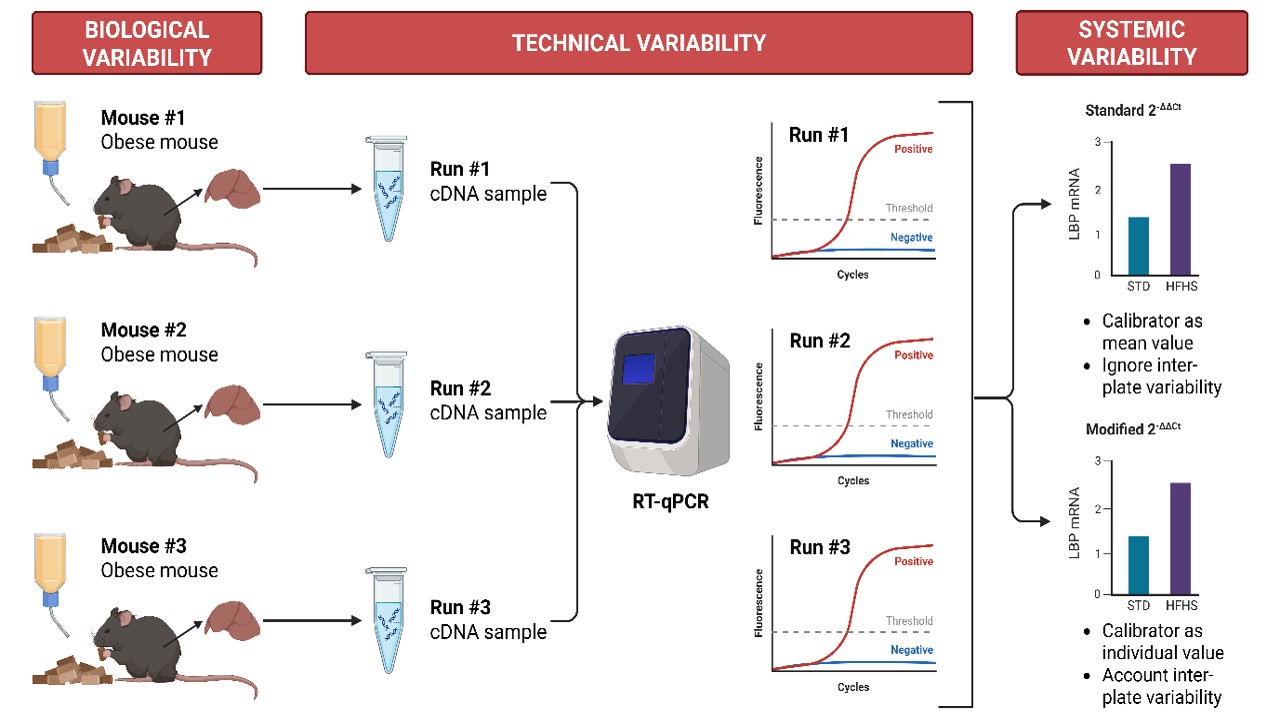
High-throughput RT-qPCR results on hepatic lipopolysaccharide-binding protein (LBP) expression in obese subjects are essential, as they reveal the endotoxin’s role in the development of obesity and non-communicable disease (NCD). This study aimed to optimize RT-qPCR detection of LBP in diet-induced obese mice. This study primarily focused on addressing high variability through reference gene normalization. A total of six male C57BL/6 mice aged 6 weeks were randomly allocated into two dietary treatments (n = 3), consisting of mice fed with the standard chow diet (SCD group) and mice fed with the high-fat and high sucrose diet (HFHS group) ad libitum for 8 weeks. Relative quantification strategies involving the standard 2-ΔΔCt method (calibrator as mean) and the modified 2-ΔΔCt method (calibrator as individual sample-matched biological replicates) were compared in terms of their variability. Obesity was successfully induced in the HFHS treatment group, as indicated by significantly higher body weight, calorie intake, and LBP relative expressions compared to the SCD group. In addition, a sample-specific calibrator approach using the modified 2-ΔΔCt method resulted in lower variability in relative gene expression levels. A modified 2-ΔΔCt method, which utilizes a sample-specific calibrator to counteract sample-specific variability, was successfully employed to address high variability in RT-qPCR results.
Clemente-Postigo, M., Oliva-Olivera, W., Coin-Aragüez, L., Ramos-Molina, B., María Giraldez-Perez, R., Lhamyani, S., Alcaide-Torres, J., Perez-Martinez, P., El Bekay, R., Cardona, F., Tinahones, F. J., & Bekay, E. R. (2019). Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. American Journal of Physiology Endocrinology Metabolism, 316, 319–332.
De Francesco, P. N., Cornejo, M. P., Barrile, F., García Romero, G., Valdivia, S., Andreoli, M. F., & Perello, M. (2019). Inter-individual variability for high fat diet consumption in inbred C57BL/6 mice. Frontiers in Nutrition, 6, 67.
de Moura e Dias, M., dos Reis, S. A., da Conceição, L. L., Sediyama, C. M. N. de O., Pereira, S. S., de Oliveira, L. L., Gouveia Peluzio, M. do C., Martinez, J. A., & Milagro, F. I. (2021). Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetology and Metabolic Syndrome, 13(1), 32.
Ehlting, C., Wolf, S. D., & Bode, J. G. (2021). Acute-phase protein synthesis: A key feature of innate immune functions of the liver. Biological Chemistry, 402(9), 1129–1145.
Fan, X., Yao, H., Liu, X., Shi, Q., Lv, L., Li, P., Wang, R., Tang, T., & Qi, K. (2020). High-Fat Diet alters the Expression of Reference Genes in Male Mice. Frontiers in Nutrition, 7, 589771.
Giri, P. S., Bharti, A. H., & Dwivedi, M. (2022). Decreased GZMB, NRP1, ITPR1, and SERPINB9 Transcripts Lead to Reduced Regulatory T Cells Suppressive Capacity in Generalized Vitiligo Patients. Journal of Immunology Research, 2022, 3426717.
Hawkins, L. J., & Storey, K. B. (2017). Improved high-throughput quantification of luminescent microplate assays using a common Western-blot imaging system. MethodsX, 4, 413–422.
Kwon, N., Lee, K. E., Singh, M., & Kang, S. G. (2021). Suitable primers for GAPDH reference gene amplification in quantitative RT-PCR analysis of human gene expression. Gene Reports, 24, 101272.
Latorre, J., Díaz-Trelles, R., Comas, F., Gavaldà-Navarro, A., Milbank, E., Dragano, N., Morón-Ros, S., Mukthavaram, R., Ortega, F., Castells-Nobau, A., Oliveras-Cañellas, N., Ricart, W., Karmali, P. P., Tachikawa, K., Chivukula, P., Villarroya, F., López, M., Giralt, M., Fernández-Real, J. M., & Moreno-Navarrete, J. M. (2022). Downregulation of hepatic lipopolysaccharide binding protein improves lipogenesis-induced liver lipid accumulation. Molecular Therapy Nucleic Acids, 29, 599–613.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4), 402–408.
McKeown, N. M., Fahey, G. C., Slavin, J., & Van Der Kamp, J. W. (2022). Fibre intake for optimal health: how can healthcare professionals support people to reach dietary recommendations? The BMJ, 11(1), e054370.
Mohammad, S., & Thiemermann, C. (2021). Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Frontiers in Immunology, 11(2), 594150.
Page, M. J., Kell, D. B., & Pretorius, E. (2022). The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress, 6(2), 24705470221076390.
Regier, N., & Frey, B. (2010). Experimental comparison of relative RT-qPCR quantification approaches for gene expression studies in poplar. http://www.biomedcentral.com/1471-2199/11/57
Seethaler, B., Basrai, M., Neyrinck, A. M., Nazare, J. A., Walter, J., Delzenne, N. M., & Bischoff, S. C. (2021). Biomarkers for assessment of intestinal permeability in clinical practice. American Journal of Physiology - Gastrointestinal and Liver Physiology, 321(1), G11–G17.
Serban, D., Mihai Branescu, C., Cristian, D. A., & Dascalu, A. M. (2020). Bilirubin, Interleukin 6 (IL 6) and Lipopolysaccharide-Binding Protein (LBP)-Biomarkers of Sepsis in Appendicular Peritonitis. Review Chim, 71(7), 444–454.
Suryadiningrat, M., Kurniawati, D. Y., Mujiburrahman, A., & Purnama, M. T. E. (2021). Dietary polyvinyl alcohol and alginate nanofibers ameliorate hyperglycemia by reducing insulin and glucose-metabolizing enzyme levels in rats with streptozotocin-induced diabetes. Veterinary World, 14(4), 847–853.
Tanaka, N., Kimura, T., Fujimori, N., Nagaya, T., Komatsu, M., & Tanaka, E. (2019). Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World Journal of Gastroenterology, 25(2), 163–177.
Teng, M. L. P., Ng, C. H., Huang, D. Q., Chan, K. E., Tan, D. J. H., Lim, W. H., Yang, J. D., Tan, E., & Muthiah, M. D. (2023). Global incidence and prevalence of nonalcoholic fatty liver disease. In Clinical and Molecular Hepatology, 29(1), 32–42.
Tjahjono, Y., Foe, K., Ratnasari Wilianto, Y., Christianto Khudrati, W., Yesery Esar, S., Jafet, N., Made Andika Bara Kusuma, I., Ade, L., Dian Novita, B., Wihadmadyatami, H., Handayani Tjan, L., Nugraha, J., Santoso, S., & Wijaya, H. (2024). HP inulin-MCT dietary fiber improves lipid metabolism and prevents non-alcoholic steatohepatitis in obese mice. Journal of Functional Foods, 120, 106367.
World Health Organization. (2023). World Health Statistics 2023: Monitoring health for the SDGs, Sustainable Development Goals.
Zhao, F., Maren, N. A., Kosentka, P. Z., Liao, Y. Y., Lu, H., Duduit, J. R., Huang, D., Ashrafi, H., Zhao, T., Huerta, A. I., Ranney, T. G., & Liu, W. (2021). An optimized protocol for stepwise optimization of real-time RT-PCR analysis. Horticulture Research, 8(1), 179.
Zhao, R., Khafipour, E., Sepehri, S., Huang, F., Beta, T., & Shen, G. X. (2019). Impact of Saskatoon berry powder on insulin resistance and relationship with intestinal microbiota in high fat–high sucrose diet-induced obese mice. Journal of Nutritional Biochemistry, 69, 130–138.
Zhu, H., Zhang, H., Xu, Y., Laššáková, S., Korabečná, M., & Neužil, P. (2020). PCR past, present and future. BioTechniques, 69(4), 317–325.
Copyright (c) 2025 Yudy Tjahjono, I Gede Putu Adhi Wedharga, Bernadette Dian Novita, Paul Tahalele, Hendy Wijaya, Endang Isbandiati Soediono, Adi Pramono Hendrata, Sianty Dewi, Sumi Wijaya, Martha Ervina, Suryo Kuncorojakti

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish in this journal agree to the following terms:
1. The journal allows the author to hold the copyright of the article without restrictions;
2. The journal allows the author(s) to retain publishing rights without restrictions;
3. The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA).






11.jpg)