BIOACTIVE COMPOUNDS FROM PURPLE ROSELLE CALYX (HIBISCUS SABDARIFFA L.) EXTRACT USING MULTISTAGE COUNTERCURRENT METHOD
Downloads
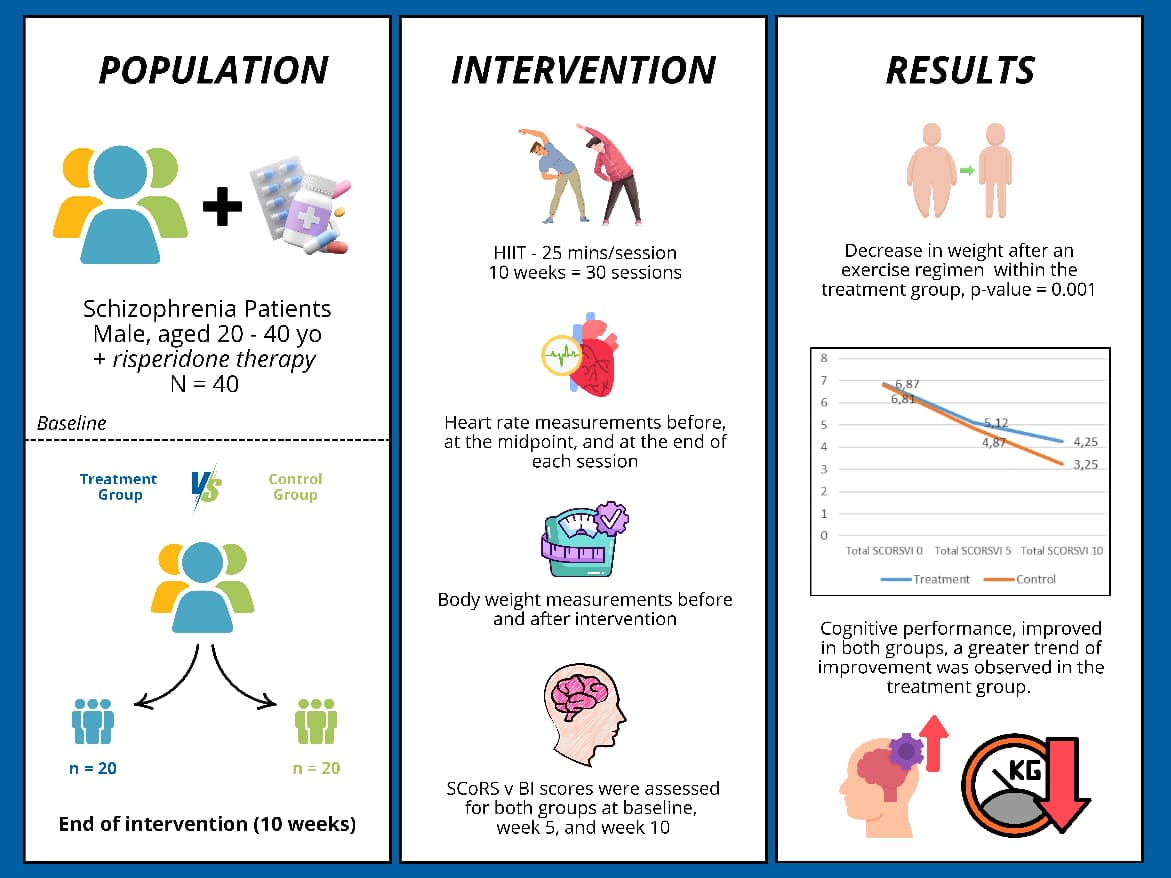
Multistage Countercurrent Extraction (MCE) is a new extraction technique used to extract bioactive compounds (anthocyanin, quercetin, antioxidants) from purple roselle calyxes (Hibiscus sabdariffa L.). This study of purple roselle calyxes extract with three-stage MCE was carried out at a comparison of roselle calyxes and distillation water solvent 1:10, extraction temperatures of 50°C, 60°C, 70°C and extraction time of 15, 30, 45 minutes. Purple roselle calyxes using the MCE method contained the highest anthocyanin content of 2815,43 mg/L, quercetin content 59,25 mg/L, and antioxidant capacity 197,6 ppm. The results showed that the content of bioactive compounds increased by increasing the extraction temperature and extraction time. MCE is an efficient technique for extracting bioactive compounds from roselle calyxes. Roselle calyxes that are rich in antioxidants have the potential as a good food colorant andnatural antioxidants.
Abdel-Aal, E. S. M., & Hucl, P. (1999). A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chemistry, 76(3), 350–354. doi:10.1094/CCHEM.1999.76.3.350
Andlauer, W., & Fürst, P. (1999). Does cereal reduce the risk of cancer? Cereal Foods World, 44(2), 76–78.
Anel, T. C., Thokchom, R., Subapriya, M. S., Thokchom, J., & Singh, S.
S. (2016). Hibiscus sabdariffa -A natural micro nutrient source. Int. J. Adv. Res. Biol. Sci. International Journal of Advanced Research in Biological Sciences, 3(4), 243–248. Retrieved from http://www.ijarbs.com/pdfcopy/apr2016/ijarbs33.pdf
Azmir, J., Zaidul, I. S. M., Rahman, M. M., Sharif, K. M., Mohamed, A., Sahena, F., Jahurul, M. H. A., Ghafoor, K., Norulaini, N. A. N., & Omar, A. K. M. (2013). Techniques for extraction of bioactive compounds from plant materials : A review. Journal of Food Engineering, 117(4), 426–436. doi:10.1016/j.jfoodeng.2013.01.014
Barhe, T. A., & Tchouya, G. R. F. (2016). Comparative study of the anti-oxidant activity of the total polyphenols extracted from Hibiscus Sabdariffa L., Glycine max L. Merr., yellow tea and red wine through reaction with DPPH free radicals. Arabian Journal of Chemistry, 9(1), 1–8. doi:10.1016/j.arabjc.2014.11.048
Bischoff, S. C. (2008). Quercetin: Potentials in the prevention and therapy of disease. Current Opinion in Clinical Nutrition and Metabolic Care, 11(6), 733–740. doi:10.1097/MCO.0b013e32831394b8
Boots, A. W., Balk, J. M., Bast, A., & Haenen, G. R. M. M. (2005). The reversibility of the glutathionyl-quercetin adduct spreads oxidized quercetin-induced toxicity. Biochemical and Biophysical Research Communications, 338(2), 923–929. doi:10.1016/j.bbrc.2005.10.031
Borrás-Linares, I., Fernández-Arroyo, S., Arráez-Roman, D., Palmeros-Suárez, P. A., Del Val-Díaz, R., Andrade-Gonzáles, I., Fernández-
Gutiérrez, A., Gómez-Leyva, J. F., & Segura-Carretero, A. (2015). Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Industrial Crops and Products, 69, 385?394-394. doi:10.1016/j.indcrop.2015.02.053
Bustos, D. V., Riegel, R., & Calderini, D. F. (2012). Anthocyanin content of grains in purple wheat is affected by grain position, assimilate availability and agronomic management. Journal of Cereal Science, 55(3), 257–264. doi:10.1016/j.jcs.2011.12.001
Calabrò, M. L., Galtieri, V., Cutroneo, P., Tommasini, S., Ficarra, P., & Ficarra, R. (2004). Study of the extraction procedure by experimental design and validation of a LC method for determination of flavonoids in Citrus bergamia juice. Journal of Pharmaceutical and Biomedical Analysis, 35(2), 349–363.doi:10.1016/S0731-7085(03)00585-5
Carvajal-Zarrabal, O., Waliszewski, S. M., Barradas-Dermitz, D. M., Orta-Flores, Z., Hayward-Jones, P. M., Nolasco-Hipólito, C., Angulo-Guerrero, O., Sánchez-Ricaño, R., Infanzón, R. M., & Trujillo, P. R. L. (2005). The consumption of Hibiscus sabdariffa dried calyx ethanolic extract reduced lipid profile in rats. Plant Foods for Human Nutrition, 60(4), 153–159. doi:10.1007/s11130-005-9023-x
Chen, W., Müller, D., Richling, E., & Wink, M. (2013). Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. Journal of Agricultural and Food Chemistry, 61(12), 3047–3053. doi:10.1021/jf3054643
Cid-Ortega, S., & Guerrero-Beltrán, J. A. (2015). Roselle calyces (Hibiscus sabdariffa), an alternative to the food and beverages industries: a review. Journal of Food Science and Technology, 52(11), 6859–6869. doi:10.1007/s13197-015-1800-9
Conklin, K. A. (2009). Dietary Antioxidants During Cancer Chemotherapy : Impact on Chemotherapeutic Effectiveness and Development of Side Effects Dietary Antioxidants During Cancer Chemotherapy : Impact on Chemotherapeutic Effectiveness and Development of Side Effects. 37(1), 1–18. doi:10.1207/S15327914NC3701
Corrales, M., Lindauer, R., Butz, P., & Tauscher, B. (2008). Effect of heat/pressure on cyanidin-3-glucoside ethanol model solutions. Journal of Physics: Conference Series, 121(PART 14). doi:10.1088/1742-6596/121/14/142003
Da-Costa-Rocha, I., Bonnlaender, B., Sievers, H., Pischel, I., & Heinrich, M. (2014). Hibiscus sabdariffa L. - A phytochemical and pharmacological review. Food Chemistry, 165, 424–443. doi:10.1016/j.foodchem.2014.05.002
Dehghan, G., & Khoshkam, Z. (2012). Tin(II)-quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chemistry, 131(2), 422–426. doi:10.1016/j.foodchem.2011.08.074
Djaeni, M., Triyastuti, M. S., Asiah, N., Annisa, A. N., & Novita, D. A. (2015). The effect of air temperature on the sappan wood extract drying. AIP Conference Proceedings, 1699. doi:10.1063/1.4938360
Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P., & Vidal, N. (2006). Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chemistry, 97(4), 654–660. doi:10.1016/j.foodchem.2005.04.028
Einbond, L. S., Reynertson, K. A., Luo, X. D., Basile, M. J., & Kennelly, E. J. (2004). Anthocyanin antioxidants from edible fruits. Food Chemistry, 84(1), 23–28. doi:10.1016/S0308-8146(03)00162-6
Giusti, M. M., & Wrolstad, R. E. (2003). Acylated anthocyanins from edible sources and their applications in food systems. Biochemical Engineering Journal, 14(3), 217–225.doi:10.1016/S1369-703X(02)00221-8
Gokmen, V., Morales, F. J., Ataç, B., Serpen, A., & Lorenzo, G. A. (2009). Multiple-stage extraction strategy for the determination of acrylamide in foods. Journal of Food Composition and Analysis, 22(2), 142–147. doi:10.1016/j.jfca.2008.09.007
Horbowicz, M., Grzesiuk, A., Debski, H., & Kosson, R. (2008). Anthocyanins of Fruits and Vegetables - Their Occurrence, Analysis and Role in Human. Vegetable Crops Research Bulletin, 68, 5–22. doi:10.2478/v10032-008-0001-8
Hosseinian, F. S., Li, W., & Beta, T. (2008). Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chemistry, 109(4), 916–924. doi:10.1016/j.foodchem.2007.12.083
Hussein, R. M., Shahein, Y. E., Hakim, A. E. El, & Awad, H. M. (2010). Biochemical and molecular characterization of three colored types of roselle (Hibiscus sabdariffa L.). Journal of American Science Org . Americanscience, 11(6), 726–733. https://www.kau.edu.sa/Files/857/Researches/58170_28293.pdf%0Ahttp://www.jofamericanscience.org/journals/am-sci/am0611/105_3886am0611_726_733.pdf
Isik, M., Korkmaz, M., Bursal, E., Gulcin, I., Koksal, E., & Tohma, H. (2015). Determination of Antioxidant Properties of Gypsophila bitlisensis Bark. International Journal of Pharmacology, 11(4), 366–371.
Jabeur, I., Pereira, E., Barros, L., Calhelha, R. C., Soković, M., Oliveira, M. B. P. P., & Ferreira, I. C. F. R. (2017). Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Research International, 100, 717–723. doi:10.1016/j.foodres.2017.07.073
Jordheim, M. (2007). Isolation, identification an properties of pyranoanthocyanins and anthocyanin forms. 98.
Kita, A., Bakowska-Barczak, A., Hamouz, K., KuÅ‚akowska, K., & LisiÅ„ska, G. (2013). The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). Journal of Food Composition and Analysis, 32(2), 169–175. doi:10.1016/j.jfca.2013.09.006
Knievel, D. C., Abdel-Aal, E. S. M., Rabalski, I., Nakamura, T., & Hucl, P. (2009). Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). Journal of Cereal Science, 50(1), 113–120. doi:10.1016/j.jcs.2009.03.007
Lee, J., Durst, R. W., & Wrolstad, R. E. (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International, 88(5), 1269–1278. doi:10.5555/jaoi.2005.88.5.1269
Liao, J., Qu, B., & Zheng, N. (2016). Effects of Process Parameters on the Extraction of Quercetin and Rutin from the Stalks of Euonymus Alatus (Thumb.) Sieb and Predictive Model Based on Least Squares Support Vector Machine Optimized by an Improved Fruit Fly Optimization Algorithm. Applied Sciences, 6(11), 340. doi:10.3390/app6110340
Liu, B., & Zhu, Y. (2007). Extraction of flavonoids from flavonoid-rich parts in tartary buckwheat and identification of the main flavonoids. Journal of Food Engineering, 78(2), 584–587. doi:10.1016/j.jfoodeng.2005.11.001
Mardiah, Zakaria, F. R., Prangdimurti, E., & Damanik, R. (2015). CHANGES IN CHEMICAL CONTENT OF RED AND PURPLE ROSELLE (Hibiscus sabdariffa L.) EXTRACTDRIED INCABINET DRYER AND FLUIDIZED BED DRYER. Jurnal Teknologi Industri Pangan, 25(1), 1–7.
Molyneux, P. (2004). The use of the stable free radical diphenylpicryl- hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin Journal of Science and Technology, 26(November 2003), 211–219. doi:10.1287/isre.6.2.144
Moon, Y. J., Wanga, L., DiCenzob, R., & Morris, M. E. (2008). Quercetin Pharmacokinetics in Humans Young. Biopharmaceutics & Drug Disposition, 29(4), 205–217. doi:10.1002/bdd
Nanjo, F., Goto, K., Seto, R., Suzuki, M., Sakai, M., & Hara, Y. (1996). Scavenging effects of tea catechins and their derivatives on 1,1- diphenyl-2-picrylhydrazyl radical. Free Radical Biology and Medicine, 21(6), 895–902. doi:10.1016/0891-5849(96)00237-7
Obadina, A. O., & Oyewole, O. B. (2007). Assessment of the antimicrobial potential of roselle juice (ZOBO) from different varieties of roselle calyx. Journal of Food Processing and Preservation, 31(5), 607–617. doi:10.1111/j.1745-4549.2007.00151.x
Oztaskin, N., Cetinkaya, Y., Taslimi, P., Goksu, S., & Gulcin, I. (2015). Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. In Bioorganic Chemistry (Vol. 60). doi:10.1016/j.bioorg.2015.04.006
Pejic, N., Kuntic, V., Vujic, Z., & Micic, S. (2004). Direct spectrophotometric determination of quercetin in the presence of ascorbic acid. Farmaco, 59(1), 21–24. doi:10.1016/j.farmac.2003.07.013
Price, K. R., Bacon, J. R., & Rhodes, M. J. C. (1997). Effect of Storage and Domestic Processing on the Content and Composition of Flavonol Glucosides in Onion ( Allium cepa ). Journal of Agricultural and Food Chemistry, 45(3), 938–942. doi:10.1021/jf9605916
Qiu, G., Wang, D., Song, X., Deng, Y., & Zhao, Y. (2018). Degradation kinetics and antioxidant capacity of anthocyanins in air-impingement jet dried purple potato slices. Food Research International, 105, 121–128. doi:10.1016/j.foodres.2017.10.050
Sanchez-Moreno, C., Larrauri, J. a., & Saura-calixto, F. (1998). A procedure to measure the antiradical efficienc y of polyphenols. Journal of the Science of Food and Agriculture, 270(199802), 270–276. doi:10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9
Sarker, S. D., & Nahar, L. (2012). An introduction to natural products isolation. Methods in Molecular Biology, 864, 1–25. doi:10.1007/978-1-61779-624-1_1
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics. CA Cancer J Clin, 66(1), 7–30. doi:10.3322/caac.21332.
Triyastuti, M. S., Kumoro, A. C., & Djaeni, M. (2017). Physical properties evaluation of roselle extract-egg white mixture under various drying temperatures. AIP Conference Proceedings, 1823. doi:10.1063/1.4978116
Tsai, P. J., McIntosh, J., Pearce, P., Camden, B., & Jordan, B. R. (2002). Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Research International, 35(4), 351–356. doi:10.1016/S0963-9969(01)00129-6
Turkmen, N., Velioglu, Y. S., Sari, F., & Polat, G. (2007). Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules, 12(3), 484–496. doi:10.3390/12030484
Vargas, F. D., & Lopez, O. P. (2003). Natural Colorants For Food and Nutraceutical for Uses. In CRC Press LLC. doi:10.1016/S0924-2244(03)00076-1
Wang, Q. E., Ma, S., Fu, B., Lee, F. S. C., & Wang, X. (2004). Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch). Biochemical Engineering Journal, 21(3), 285–292. doi:10.1016/j.bej.2004.06.002
Wong, C. C., Li, H. Bin, Cheng, K. W., & Chen, F. (2006). A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chemistry, 97(4), 705–711. doi:10.1016/j.foodchem.2005.05.049
Yu, C. H., Chen, J., Xiong, Y. K., Li, X. X., Dai, X. Y., & Shi, C. C. (2012). Optimization of multi-stage countercurrent extraction of antioxidants from Ginkgo biloba L. leaves. Food and Bioproducts Processing, 90(2), 95–101. doi:10.1016/j.fbp.2011.05.003
Yue, X., & Xu, Z. (2008). Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. Journal of Food Science, 73(6), 494–499. doi:10.1111/j.1750-3841.2008.00845.x
Zhang, Q., Bian, Y., Shi, Y., Zheng, S., Gu, X., Zhang, D., Zhu, X., Wang, X., Jiang, D., & Xiong, Q. (2015). An economical and efficient technology for the extraction of resveratrol from peanut (Arachis hypogaea) sprouts by multi-stage countercurrent extraction. Food Chemistry, 179, 15–25. doi:10.1016/j.foodchem.2015.01.113
- MEDIA GIZI INDONESIA Journal is the copyright owner of all materials published on this website.
- The formal legal provisions for access to digital articles of this electronic journal are subject to the terms of the Creative Commons Attribution-NonCommercial-ShareAlike license (CC BY-NC-SA 4.0), which means that MEDIA GIZI INDONESIA Journal and readers reserve the right to save, transmit media / format, manage in database, maintain, and publish articles as long as it continues to include the name of the Author.
- Printed and published print and electronic manuscripts are open access for educational, research and library purposes. In addition to these objectives, the editorial board shall not be liable for violations of copyright law.


2.png)