Effect of Epigallocatechin Gallate on the Inflammatory Response in Mice (Mus musculus) Kidneys
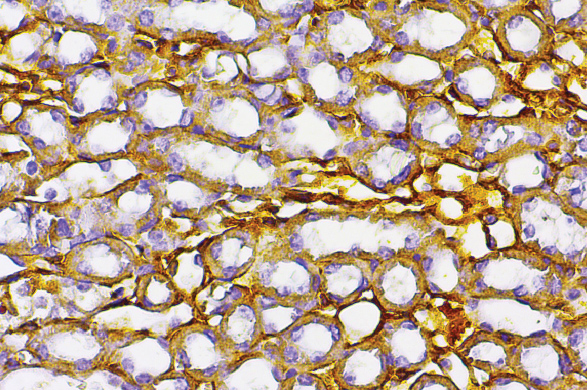
Background: Epigallocatechin gallate is the primary polyphenol constituent of green tea. It has the ability to inhibit the pathological processes caused by oxidants. However, in certain cases, the green tea diet is known to exert pro-oxidant effects. In addition, studies have shown that epigallocatechin gallate negatively affects cells. Several studies on epigallocatechin gallate showed increased oxidative stress and decreased intracellular antioxidants. Furthermore, it can stimulate an inflammatory response from the innate immune system, which may contribute to the elimination of the effects of epigallocatechin gallate. Purpose: This study aims to investigate the inflammatory responses (IL-1, IL-6, and TNF-α) in mice kidneys due to epigallocatechin gallate. Methods: This study involved the use of experimental animals aged between two and three months with an average body weight of 20 grams. The animals were randomly divided into two groups, namely the control group and the epigallocatechin gallate treatment group, with each group consisting of 16 samples. The dose of epigallocatechin gallate used in this study was 750 mg/kg bw. The treatment was administered for three days, after which the kidneys were collected. Immunohistochemical staining was used to observe the inflammatory response, including IL-1, IL-6 and TNF-α. Subsequently, all the data were collected and statistically analyzed using an independent t-test. Results: The results of the data analysis showed a significant difference in the expression of IL-6 (p = 0.018) and TNF-α (p = 0.000), but no significant difference in the expression of IL-1 (p = 0.106). Conclusion: In conclusion, epigallocatechin gallate was found to induce an inflammatory response in mice kidneys.
Abbas, A.K., Andrew, H.L., and Shiv, P., 2022. Cellular and Molecular Immunology. 10th Edition. Pennsylvania: Elsevier.
Billingham, L.K., Stoolman, J.S., and Vasan, K., 2022. Mitochondrial Electron Transport Chain is Necessary for NLRP3 Inflammasome Activation. Nature Immunology, 23, 692–704.
Chi, W., Hongrui, C., Fei, L., Yingting, Z., Wei, Y., and Yehong, Z., 2015. HMGB1 Promotes the Activation of NLRP3 and Caspase-8 Inflammasomes via NF-kB Pathway in Acute Glaucoma. Journal of Neuroinflammation, 12, 1-12.
England, H., Hazel, R.S., Michelle, E.E., Nancy, J.R., and David, B., 2014. Release of Interleukin-IL-1α and Interleukin-1β Depends on Mechanism of Cell Death. Journal of Biological Chemistry, 289, 15942-15950.
Evavold, C. L., and Jonathan, C. K., 2019. Inflammasomes: Threat-Assessment Organelles of the Innate Immune System. Immunity Review, 51, 677-687.
He, M., Marco, E.B., Tom, R.C., Kevin, J.T., and Yousef, A.A., 2018. Exploring the Biological Functional Mechanism of the HMGB1/TLR4/MD-2 Complex by Surface Plasmon Resonance. Molecular Medicine, 24, 1-9.
James, K.D., Mary, J.K., and Joshua, D.L., 2018. Potential Role of the Mitochondria as a Target for the Hepatotoxic Effects of (-)-epigallocatechin-3-gallate in Mice. Food and Chemical Toxicology, 111, 302-309.
Khan, H. A., Khalid E. I., Sara T. A., Salman A., Salman H. A., Najla A., Mohsen G. A., Samia H. S., and Isra K., 2020. Immunohistochemistry of IL-1β, IL-6 and TNF-α in Spleens of Mice Treated with Gold Nanoparticles. Saudi Journal of Biological Sciences, 27(4), 1163-1168.
Lambert, J.D., Mary, J.K., Shengmin, S., Kenneth, R.R., Jihyeung, J., and Chung, S.Y., 2010. Hepatotoxicity of High Oral Dose(-)-epigallocatechin-3-gallate in Mice. Food and Chemical Toxicology Journal, 48, 409-416.
Meng, X.Y., Li, B., Liu, S., Kang, H., Zhao, L., and Zhou, R., 2016. EGCG in Green Tea Induces Aggregation of HMGB1 Protein through Large Conformational Changes with Polarized Charge Redistribution. Scientific Reports, 6, 1-10.
Merle, N.S., Remi, N., Lise, H.M., Veronique, F.B., and Lubka, T.R., 2015. Complement System Part II: Role in Immunity. Frontiers Immunology, 6, 1-26.
Mitroulis, I., Vasileia, I.A., Ioannis, K., Athanassios, Z., George, H., and Triantafyllos, C., 2015. Leukocyte Integrins: Role in Leukocyte Recruitment and as Therapeutic Targets in Inflammatory Disease. Pharmacology and Therapeutics, 147, 123-135.
Murphy, K., and Casey, W., 2017. Janeway's Immunobiology. 9th Edition. New York: Garland Science.
Puledran, B., 2024. Integrated Organ Immunity: a Path to a Universal Vaccine. Nature Reviews Immunology, 24, 81-82.
Sotler, R., Borut, P., Raja, D., Tomislav, J., Doroteja, P.J., Cecilija, R., Polonca, T., and Andrej, S., 2019. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clina Croatica, 58(4), 726-736.
Varghese, F., Amirali, B.B., Renu, M., and Abhijit, D., 2014. IHC-Profiler: An Open-Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLOS One, 9(5), 1-11.
Virag, L., Rafael, I.J., Zsolt, R., Lisardo, B., and Patricia, P., 2019. Self-Defense of Macrophages Against Oxidative Injury: Fighting for Their Own Survival. Redox Biology, 26, 1-9.
Yang, H., Haichao, W., and Ulf, A., 2020. Targeting Inflammation Driven by HMGB1. Frontiers Immunology, 11, 1-9.
Zachary, J.F., 2017. Pathology Basis of Veterinary Disease 6th Edition. China: Elsevier.
Zheng, D., Liwinski, T., and Elinav, E., 2020. Inflammasome Activation and Regulation: Toward a Better Understanding of Complex Mechanisms. Cell Discovery, 6, 1-22.
Copyright (c) 2024 Authors

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
- The journal allows the author to hold the copyright of the article without restrictions.
- The journal allows the author(s) to retain publishing rights without restrictions.
- The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution Share-Alike (CC BY-SA).

Journal of Applied Veterinary Science and Technology is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License