COMPARISON OF MORTALITY BETWEEN THE COMBINATION OF STANDARD THERAPY AND CONVALESCENT PLASMA THERAPY AND STANDARD THERAPY ONLY IN COVID-19 PATIENTS : SYSTEMATIC REVIEW AND META-ANALYSIS
Downloads
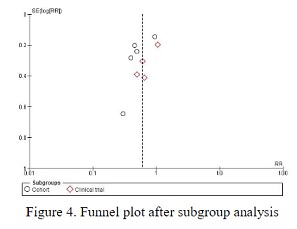
Coronavirus Disease-19 (COVID-19) is an infectious disease caused by a newly discovered type of coronavirus. Comprehensive management for COVID-19 patients includes infection control, hemodynamic stability maintenance, oxygenation monitoring, ventilation, and pharmacotherapy administration. Convalescent plasma is one of the COVID-19 therapy choices, proven to provide relief for Ebola, SARS, and MERS patients. Therefore, the authors believed in searching data on whether convalescent plasma therapy also improves COVID-19 patients, specifically in terms of mortality. This study aims to compare the comparison in mortality between standard therapy and convalescent plasma therapy with standard therapy only in COVID-19 patients. This study used a systematic review and meta-analysis method according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. This study used ten studies that met the inclusion criteria to evaluate the comparison in mortality between the combination therapy with standard therapy only in COVID-19 patients. There was a significant difference in mortality between the combination of standard therapy and convalescent plasma therapy with standard therapy only in COVID-19 patients, and mortality in the combination therapy groups being lower than standard therapy only.
WHO (2021). Indonesia: WHO Coronavirus (COVID-19) Dashboard, https://covid19.who.int/region/searo/country/id (Accessed 25 April 2021)
NIH (2020). COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health
(PDPI) PDPI, (PERKI) PDSKI, (PAPDI) PDSPDI, et al (2020). Pedoman Tatalaksana COVID-19 edisi 2
RI K (2020). Gugus Tugas Percepatan Penanganan COVID-19, https://covid19.go.id/peta-sebaran
Li L, Zhang W, Hu Y, et al (2020). Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. Jama, 324, 460-470. DOI: 10.1001/jama.2020.10044.
Agarwal A, Mukherjee A, Kumar G, et al (2020). Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). Bmj, 371, m3939 DOI: 10.1136/bmj.m3939.
Abolghasemi H, Eshghi P, Cheraghali AM, et al (2020). Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apher Sci, 59, 102875. DOI: 10.1016/j.transci.2020.102875.
Allahyari A, Seddigh-Shamsi M, Mahmoudi M, et al (2021). Efficacy and safety of convalescent plasma therapy in severe COVID-19 patients with acute respiratory distress syndrome. International immunopharmacology, 93, 107239-107239.
DOI: 10.1016/j.intimp.2020.107239.
Shenoy AG, Hettinger AZ, Fernandez SJ, et al (2021). Early mortality benefit with COVID-19 convalescent plasma: a matched control study. Br J Haematol, 192, 706-713. DOI: 10.1111/bjh.17272.
Salazar E, Christensen PA, Graviss EA, et al (2021). Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG. The American journal of pathology, 191, 90-107
DOI: 10.1016/j.ajpath.2020.10.008.
Alsharidah S, Ayed M, Ameen RM, et al (2021). COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: A multicenter interventional study. Int J Infect Dis,103: 439-446. 2020/12/08.
DOI: 10.1016/j.ijid.2020.11.198.
Tworek A, Jaroń K, Uszyńska-Kałuża B, et al (2021). Convalescent plasma treatment is associated with lower mortality and better outcomes in high-risk COVID-19 patients - propensity-score matched case-control study. Int J Infect Dis, 105, 209-215. DOI: 10.1016/j.ijid.2021.02.054.
Budhiraja S, Dewan A, Aggarwal R, et al (2021). Effectiveness of convalescent plasma in Indian patients with COVID-19. Blood Cells Mol Dis, 88, 102548.
DOI: 10.1016/j.bcmd.2021.102548.
Salazar E, Christensen PA, Graviss EA, et al (2020). Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am J Pathol, 190, 2290-2303. DOI: 10.1016/j.ajpath.2020.08.001.
Putera DD, Hardianti MS (2020). Efficacy and safety of convalescent plasma therapy in patients with COVID-19: arapid review of case series. J Med Sci, 52(3), 134-147. DOI: 10.19106/JMedSciSI005203202012.
Hu X, Hu C, Jiang D, et al (2020). Effectiveness of Convalescent Plasma Therapy for COVID-19 Patients in Hunan, China. Dose-Response, 18, 1559325820979921.
DOI: 10.1177/1559325820979921.
Gazitúa R, Briones JL, Selman C, et al (2020). Convalescent Plasma in COVID-19. Mortality-Safety First Results of the Prospective Multicenter FALP 001-2020 Trial. DOI:10.1101/2020.11.30.20218560.
Copyright (c) 2021 Felicia Klarin, Angelica Diana Vita, Cynthia Elvira Sari Siahaan, Salsabila Nabilah Rifdah, Ayu Rahmanita Putri, Indriasti Putri Kusuma, Subur Prayitno

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
- The journal allows the author to hold the copyright of the article without restrictions.
- The journal allows the author(s) to retain publishing rights without restrictions.
- The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution Share-Alike (CC BY-SA).
- The Creative Commons Attribution Share-Alike (CC BY-SA) license allows re-distribution and re-use of a licensed work on the conditions that the creator is appropriately credited and that any derivative work is made available under "the same, similar or a compatible license”. Other than the conditions mentioned above, the editorial board is not responsible for copyright violation.