Antimalarial Activity of n-Hexane, n-Butanol Fractions of Spilanthes filicaulis in Swiss Mice Infected with Plasmodium berghei
Downloads
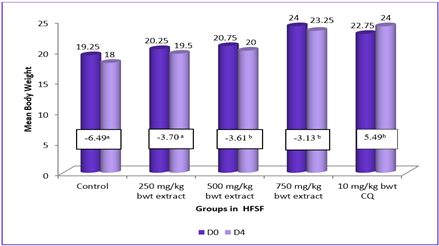
Malaria, a serious disease that can be fatal if left untreated, is caused by Plasmodium parasites. Malaria poses a significant life threat due to growing parasite resistance to drugs and the prohibitive cost of treatment, particularly in high-prevalence African countries. The prospect offered by the exploration of botanicals as alternatives necessitated this study to examine the antimalarial activities of the n-hexane fraction of Spilanthes filicaulis (HFSF) and its butanol fraction (BFSF) on Plasmodium berghei-infected mice. Swiss mice of both genders were infused with a chloroquine-sensitive P. berghei (NK-65) strain intraperitoneally. Antimalarial activity (in vivo) of S. filicaulis fractions was evaluated against early and established infection employing 4-day suppressive and curative antimalarial models at 250, 500 and 750 mg/kg dose levels respectively. Rectal temperature (RT), packed cell volume (PCV), body weight (BW), parasitemia level and mean survival time (MST) were variables determined. Findings herein demonstrated marked prevention of BW, RT, and PCV reduction at the treated doses relative to the untreated controls. Moreover, both fractions significantly suppressed parasitemia dose-dependently. The highest antimalarial chemosuppression was demonstrated by the HFSF producing 60.59%, 69.29% and 71.17% inhibition in the 4-day suppression and the BFSF yielded 45.44%, 43.96% and 47.97% chemosuppression in the curative, at the treated doses respectively. Similarly, the fractions delayed the mean survival duration of treated infected mice relative to the untreated group. Therefore, the results herein suggest that both fractions demonstrate dose-dependent and statistically significant suppression of parasitemia and improved clinical parameters in mice infected with P.berghei against murine malaria.
Aarthi, N. and Murugan, K. (2011) ‘Antimalarial activity and phytochemical screening of ethanolic leaf extract of Phyllanthus niruri and Mimosa pudica,’ International journal of pharmaceutical research and development, 3(8), pp. 198-205.
Adeleke, M.T.V. and Ndah, S. (2022) ‘Comparative phytochemical analysis of four medicinal plants traditionally used for malaria therapy in Nigeria,’ World Journal of Biology Pharmacy and Health Sciences, 10(01), pp. 080–085, Available at: http://dx.doi.org/10.30574/wjbphs.2022.10.1.0069
Ademoye, M.A., Lajide, L., and Owolabi B.J. (2018) ‘Phytochemical and Antioxidants Screening of Chrysophyllum albidum, Mezoneuron benthamianum, Phyllanthus muellerianus and Acalypha fimbriata,’ International journal of science, 7(11), pp. 8-18, Available at: http://dx.doi.org/10.18483/ijSci.1803.
Adesina, D.A. et al. (2020) ‘Antiplasmodial effect and sub-acute toxicity of alkaloid, flavonoid and phenolic extracts of Sida acuta leaf on Plasmodium berghei-infected animals’, Journal of Taibah University for Science, 14(1), pp. 943–953, Available at: https://doi.org/10.1080/16583655.2020.1790912
Agbafor, K. et al. (2015) ‘Antioxidant Activities of Ethanoilc Extractsof Spilanthes uliginosa, Ocimum basilicum, Hyptis spicigera and Cymbopogon citratusagainst Swiss Mice Exposed to Plasmodium berghei Anka 65’, American Journal of Plant Sciences, 6(1), pp. 64-72, Available at: http://dx.doi.org/10.4236/ajps.2015.61008
Akinwunmi, K.F., Ofeniforo, B.E., and Omisore, N.O. (2018) ‘Evaluation of Antioxidant, Anti Inflammatory and Antimalarial Activities of the Methanolic Leaf Extract of Spilanthes filicaulis (Schumach & Thonn)’, Journal of pharmaceutical biology, 8(1), pp. 1-11, Available at: https://doi.org/10.1089/vbz.2024.0039
Alaribe, C. et al. (2020) ‘Suppressive and curative antiplasmodial properties of Nauclea latifolia root extract and fractions against erythrocytic stage of mice-infective chloroquine-sensitive Plasmodium berghei NK-65’, Journal of Medicinal Plants for Economic Development, 4(1), pp. 1-6, Available at: http://dx.doi.org/10.4102/jomped.v4i1.72
Amelo, W., Nagpal, P. and Makonnen, E. (2014) ‘Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaea angustifolia in Plasmodium berghei infected mice’, BMC Complementary and Alternative Medicine, 14(1), pp. 462, Available at: http://dx.doi.org/10.1186/1472-6882-14-462.
Asrade, S. (2017) ‘In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against Plasmodium berghei’, Journal of Experimental Pharmacology, 9, pp. 59–66, Available at: http://dx.doi.org/10.2147/JEP.S130491
Balogun, E. et al. (2010) ‘Activity of ethanolic extract of Clerodendrum violaceum leaves against Plasmodium berghei in mice’, Agriculture and Biology Journal of North America, 1(3), pp. 307–312, Available at: http://dx.doi.org/10.5251/abjna.2010.1.3.307.312
Balogun, E. et al. (2012) ‘Biochemical and histological changes associated with treatment of malaria and diabetes mellitus in mice with extracts of Mormodiaca charantia’, An International Journal of the Nigerian Society for Experimental Biology, 24(I), pp. 38–47
Bantie, L. et al. (2014) ‘In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht (Euphorbiaceae) against Plasmodium berghei in mice’, BMC Complement Altern Med, 14(79), Available at: http://dx.doi.org/10.1186/1472-6882-14-79.
Belay, W., Gurmu, A., and Wubneh, Z. (2018) ‘Antimalarial activity of stem bark of P. linearifolia during early and established Plasmodium infection in mice’, Evidence-Based Complem & Alt Med, 2018(1), pp. 1-7, Available at: http://dx.doi.org/10.1155/2018/4169397
Chen, Y.F. et al. (2010) ‘Foam properties and detergent abilities of the saponins from Camellia oleifera’, Inernational Journal of Molecular Science, 11, pp. 4417–4425, Available at: https://doi.org/10.3390/ijms11114417
Das, S. et al. (2022) ‘Quantitative estimation of Terpenoid content in some Tea Cultivars of North East India and Their in vitro Cell Cultures using an optimized Spectrophotometric method’, Journal of Advanced Scientific Research, 13(03), pp. 112-117. Available at: https://doi.org/10.55218/JASR.202213318
Devi, C.B. et al. (2019) ‘Effect of drying procedures on nutritional composition, bioactive compounds and antioxidant activity of wheatgrass (Triticum aestivum L)’, Journal of Food Science and Technology, 56(1), pp. 491-496. Available at: https://doi.org/10.1007/s13197-018-3473-7
Ibukunoluwa, M.R. (2017) ‘In vivo antiplasmodial activity and histopathological analysis of water and ethanol extracts of a polyherbal antimalarial recipe’, Journal of Pharmacognosy and Phytotherapy, 9(6), pp. 87-100, Available at: http://dx.doi.org/10.5897/JPP2017.0449
Jacob, J. et al. (2016) ‘Nutritional composition of selected wild fruits from Minna Area of Niger State, Nigeria’, International Journal of Food Science and Biotechnology, 10(1), pp. 37–42.
Jayaraj, P., Raghavan, G. and Pushpangadan P. (2013) ‘The Genus Spilanthes Ethnopharmacology, Phytochemistry, and Pharmacological Properties’, A Review. Advances in Pharmacological Sciences, pp. 1-23, Available at: http://dx.doi.org/10.1155/2013/510298
Jothy, S. et al. (2011) ‘Acute oral toxicity of methanolic seed extract of Cassia fstula in mice’, Molecules, 16(6), pp. 5268–5282, Available at: http://dx.doi.org/10.3390/molecules16065268.
Kumar, S. et al. (2017) ‘Antiplasmodial potential and quantification of aloin and aloe-emodin in Aloe vera collected from different climaticregions of India’, BMC Complementary and Alternative Medicine, 17(1), pp. 369. Available at: https://doi.org/10.1186/s12906-017-1883-0
Mace, K. et al. (2015) ‘Evaluation of sulphadoxin–pyrimethamine for intermittent preventive treatment of malaria in pregnancy, a retripetive birth outcome study in Mansa’, Malaria Journal, 14(69), pp. 1–11, Available at: http://dx.doi.org/10.1186/s12936-015-0576-8
Madaan, R. et al. (2011) ‘Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies’, Indian Journal of Pharmaceutical Sciences,73(6), pp. 666. Available at: https://doi.org/10.4103/0250-474X.100242
Madara, A. et al. (2010) ‘Anti-malarial activity of ethanolic leaf extract of Piliostigma thonningii Schum. (Caesalpiniaceae) in mice infected with Plasmodium berghei’, Afr J Biotechnology, 9, pp. 3475–3480. Available at: http://www.academicjournals.org/AJB
Mengiste, B., Makonnen, E., and Urga, K. (2012) ‘Invivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model’, Momona Ethiopian Journal of Science, 4(1), pp. 47-63, Available at: https://doi.org/10.4314/mejs.v4i1.74056
Merlin, L.K.M. et al. (2019) ‘Toxicity and Safety Implications of Herbal Medicines Used in Africa’, Intechopen Herbal Medicine, Available at: http://dx.doi.org/10.5772/intechopen.72437
Misganaw, D., Amare, G.G., and Mengistu, G. (2020) ‘Chemo Suppressive and Curative Potential of Hypoestes forskalei against Plasmodium berghei: Evidence for in vivo Antimalarial Activity’, Journal of Experimental Pharmacology, 2020(12), pp. 313–323, Available at: http://dx.doi.org/10.2147/JEP.S262026
Ngo, T. et al. (2017) ‘Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L.’, Journal of Food Quality, 2017(1), pp. 1-8, Available at http://dx.doi.org/10.1155/2017/9305047
Nureye, D. et al. (2018) ‘In vivo antimalarial activity of 80% methanol root bark extract and solvent fractions of Gardenia ternifolia Schumach & Thonn (Rubiaceae) against Plasmodium berghei infected mice’, Evidence-Based Complementary and Alternative Medicine, 2018(1), pp. 1-10, Available at: http://dx.doi.org/10.1155/2018/9217835
Ofeniforo, B. et al. (2024a) ‘Assessing the Oxidative Stress Reducing Potential of Spilanthes flicaulis (Schumach & Thonn) Ethyl Acetate Sub fractions on Plasmodium berghei Infected Female Mice,’ Acta Parasitologica, 2024(69), pp. 1990-1997, Available at: https://doi.org/10.1007/s11686-024-00925-9
Ofeniforo, B. et al. (2024b) ‘Phytochemical analysis and in vivo antimalarial activities of ethyl acetate fraction of Spilanthes flicaulis on mice subjected to Plasmodium berghei,’ Vector-Borne Zoonotic Disease, 25(1), pp. 26-33, Available at: https://doi.org/10.1089/vbz.2024.0039
Olanlokun, J.O., Balogun, A.A., and Olorunsogo, O.O. (2021) ‘Influence of Artesunate Combinative Therapy CoAdministration with Rutin on Inflammatory Cytokines and Immunoglobulins in Plasmodium berghei Infected Mice’, Journal of Parasitology, 107(4), pp. 639–647, Available at: http://dx.doi.org/10.1645/20-87
Olorunniyi, O. (2013) ‘In vivo antimalarial activity of crude aqueous bark extract of Trichilia monadelpha against Plasmodium berghei berghei (NK65) in mice,’ International Journal of Pharmaceutical and Bio Medical Science, 2(4), pp. 2278–5221.
Opajobi, A. O. et al. (2022) ‘Inhibition of Plasmodium berghei growth by alkaloid extract of Phyllanthus amarus in mice increased haem level and stabilized erythrocyte membrane’, International Journal of Health Sciences, 6(S2), pp. 13028–13043. Available at: https://doi.org/10.53730/ijhs.v6nS2.8427
Osuntokun, O.T. and Faniyi, I.T. (2020) ‘Curative, Suppressive and Prophylactic Phyto-Medicinal Therapy/Synergistic Efficacy of Alstonia boonei/Capsicum frutescens Ethanolic Extracts against Plasmodium berghei (NK 65)/Salmonella typhi (ATCC 35723) Infected Swiss Albino Mice’, Asian Journal of Biotechnology and Genetic Engineering, 3(2), pp. 19-34.
Oyawaluja, B.O. et al. (2024) ‘Antioxidant Profiling, Phytochemical Investigation and Pharmacognostic Evaluation of Nephrolepis biserrata (SW.) Schott (Nephrolepidaceae)’, Tropical Journal of Phytochemistry and Pharmaceutical Sciences, 3(2):208-215. Available at: http://www.doi.org/10.26538/tjpps/v3i2.8
Qaiser, A. et al. (2025) ‘Evaluation of Hematological changes and types of anaemias in Malaria,’ JPTCP, 32(4), pp. 466-471. Available at: https://doi.org/10.53555/hmjkew89
Senthilkumaran, P. et al. (2024) ‘Qualitative phytochemical analysis of medicinal plants selected from Kolli hills Namakkal,’ Journal of Medicinal Plants Studies, 12(3), pp. 171-173.
Shubhra, R. et al. (2019) ‘Investigation of Phytoconstituents and Antioxidants activity of Diospyros malabarica fruit extracts’, Advances in Bioscience and Biotechnology, 10(12), pp. 431-454, Available at: http://dx.doi.org/10.4236/abb.2019.1012031
Taherkhani, M. (2013) ‘Comparison of antimalarial activity of Artemisia turanica extract with current drugs in vivo’, Journal of Vector Borne Diseases, 50(1), pp. 51-56.
Vanaja, P. et al. (2025) ‘Metabolite profiling, antimalarial potentials of Schleichera oleosa using LC-MS and GC-MS: in vitro, molecular docking and molecular dynamics’, Frontiers in Molecular Biosciences, 12:1543939. Available at: https://doi.org/10.3389/fmolb.2025.1543939
Wondafrash, D.Z. (2019) ‘Antimalarial Activity of Cordia africana (Lam.) (Boraginaceae) Leaf Extracts and Solvent Fractions in Plasmodium berghei-Infected Mice’, Evidence-Based Complementary and Alternative Medicine, 2019(1), pp. 1-14, Available at: http://dx.doi.org/10.1155/2019/8324596
World Health Organization (2020) Key facts World Malaria Report 2020. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020
Zareen, S. et al. (2020) ‘Antiplasmodial potential of Eucalyptus oblique leaf methanolic extract against Plasmodium vivax:An in vitro study’, De Gruyter Open Chemistry, 19, pp. 1023–1028. Available at: https://doi.org/10.1515/chem-2021-0091
Copyright (c) 2025 Journal of Parasite Science

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
- Every manuscript submitted to must observe the policy and terms set by the Journal of Parasite Science
- Publication rights to manuscript content published by the Journal of Parasite Science is owned by the Journal of Parasite Science with the consent and approval of the author(s) concerned
- Authors and other parties are bound to the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License for the published articles, legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA)
- By submitting the manuscript, the author agrees to the requirement that the copyright of the submitted article will be transferred to Journal of Parasite Science as the publisher of the journal. The intended copyright includes the right to publish articles in various forms (including reprints). journal of parasite science retains the publishing rights to published articles.