Trypanosoma evansi as a Major Cause of Animal Trypanosomiasis: A Comprehensive Review
Downloads
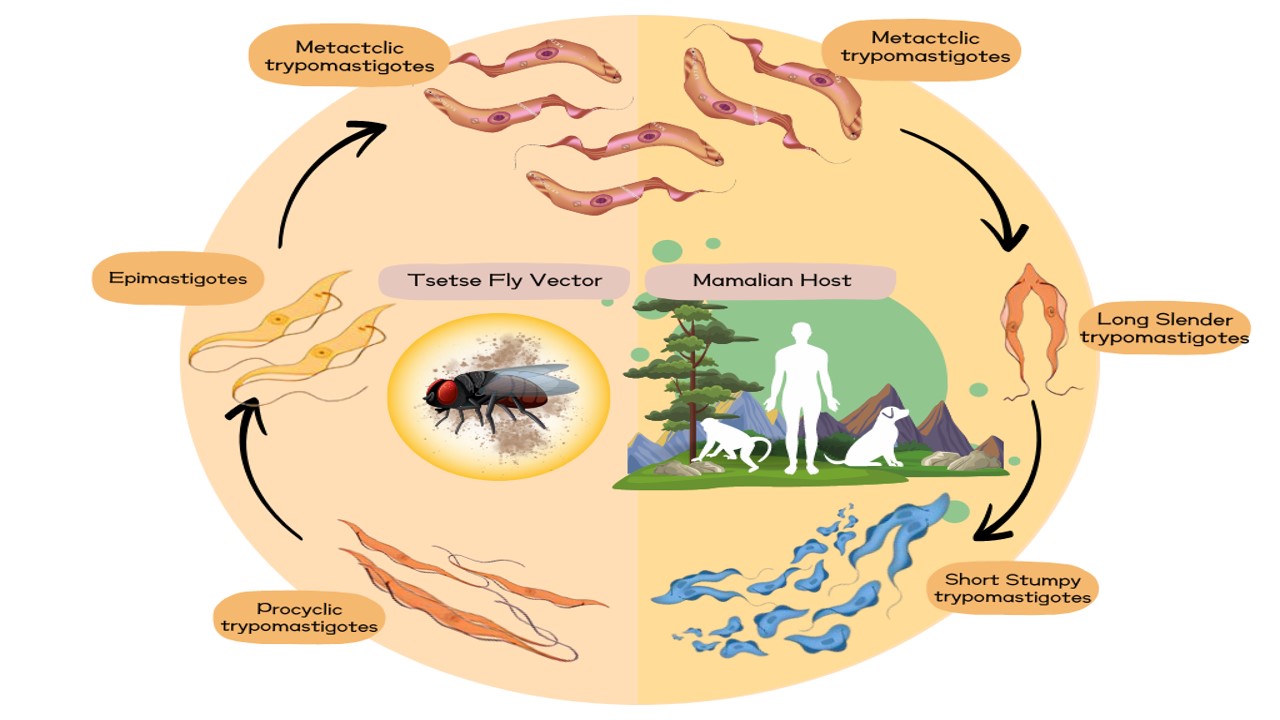
Trypanosomiasis caused by Trypanosoma evansi is a major protozoan illness that affects animals worldwide. It is also referred to as “surra” and affects a variety if wild and domestic animals such as sheep, cattle, goats, dogs, buffaloes, pigs, elephants, amongst others. In preparing this review, relevant scientific articles were searched on PubMed, SCOPUS, and Web of Science databases using the keyword “Trypanosoma evansi AND animals”. T. evansi are carried by a vast number of hematophagous flies and are found in the extracellular and internal fluids of certain hosts. Trypanosomosis is mostly characterized by anemia, and the degree of anemia can typically be used as a gauge for the disease's severity. Trypanosomiasis compromises the host animal's immune system and its diagnosis is dependent on a number of factors such as thorough clinical examination, suitable sample collection, sample size, suitable diagnostic test performance, and logical interpretation of test results. The clinical manifestations of trypanosomiasis vary widely in both appearance and severity, ranging from neurological disturbances and skin plaques to vaginal enlargement. Hematophagous biting flies, including Tabanus, Haematopota, Glossina, Chrysops, Lyperosia, Stomoxys, and Hippobusca flies, contribute to the spread of trypanosomiasis. Four medications are primarily used to treat trypanosomiasis: quinapyramine, karetin, diminazene aceturate (Berenil), and melarsomine (cymelarsan). An efficient vaccination program is an additional technique for managing infectious diseases in addition to treatment. The most important step in curtailing the spread of trypanosomiasis caused by T. evansi is to stop its transmission by flies via physical and chemical methods.
Abdel-Rady, A. (2008). Epidemiological studies (parasitological, serological and molecular techniques) of Trypanosoma evansi infection in camels (Camelus dromedarius) in Egypt. Veterinary World, 1(11), 325–328.
Abera, A., Mamecha, T., Abose, E., Bokicho, B., Ashole, A., Bishaw, T., Mariyo, A., Bogale, B., Terefe, H., Tadesse, H., Belachew, M., Difabachew, H., Eukubay, A., Kinde, S., Ali, A., Regasa, F., Seife, F., Kebede, Z., Wossen, M., Tollera, G., Hailu, M., Manaye, N., Van Reet, N., Priotto, G., van Griensven, J., Pareyn, M., & Tasew, G. (2024). Reemergence of Human African Trypanosomiasis Caused by Trypanosoma brucei rhodesiense, Ethiopia. Emerging Infectious Disease, 30(1), 125-128.
Abro, Z., Kassie, M., Muriithi, B., Okal, M., Masiga, D., Wanda, G., Gisèle, O., Samuel, A., Nguertoum, E., Nina, R.A., Mansinsa, P., Adam, Y., Camara, M., Olet, P., Boucader, D., Jamal, S., Garba, A.R.I., Ajakaiye, J.J., Kinani, J.F., Hassan, M.A., Nonga, H., Daffa, J., Gidudu, A., & Chilongo, K. (2021). The potential economic benefits of controlling trypanosomiasis using waterbuck repellent blend in sub-Saharan Africa. PLOS ONE, 16(7), e0254558.
Adebiyi, O. E., Omobowale, T. O., & Abatan, M. O. (2021). Neurocognitive domains and neuropathological changes in experimental infection with Trypanosoma brucei brucei in Wistar rats. Heliyon, 7(11), e08260.
Aksoy, S. (2019). Tsetse peritrophic matrix influences for trypanosome transmission. Journal of Insect Physiology, 118(1), 103919.
Aksoy, S., Buscher, P., Lehane, M., Solano, P., & Van Den Abbeele, J. (2017). Human African trypanosomiasis control: Achievements and challenges. PLOS Neglected Tropical Diseases, 11(4), e0005454.
Alfituri, O. A., Quintana, J. F., MacLeod, A., Garside, P., Benson, R A., Brewer, J. M., Mabbott, N. A., Morrison, L. J., & Capewell, P. (2020). To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host. Frontiers in Immunology, 11(1), 1250.
Aregawi, W. G., Agga, G. E., Abdi, R. D., & Büscher, P. (2019). Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasites & Vectors, 12(1), 67.
Aresta-Branco, F., Sanches-Vaz, M., Bento, F., Rodrigues, J. A., & Figueiredo, L. M. (2019). African trypanosomes expressing multiple VSGs are rapidly eliminated by the host immune system. Proceedings of the National Academy of Sciences USA, 116(41), 20725–20735.
Austen, J. M., & Barbosa, A. D. (2021). Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective. Pathogens, 10(9), 1148.
Baker, N., de Koning, H. P., Mäser, P., & Horn, D. (2013). Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends in Parasitology, 29(3), 110–118.
Baral, T. N. (2010). Immunobiology of African trypanosomes: need of alternative interventions. Journal of Biomedicine and Biotechnology, 2010(1), 389153.
Barrett, M. P., & Croft, S. L. (2012). Management of trypanosomiasis and leishmaniasis. British Medical Bulletin, 104(1), 175–196.
Behour, T. S., Aboelhadid, S. M., Mousa, W. M., Amin, A. S., & El-Ashram, S. A. (2019). Molecular diagnosis of acute and chronic infection of Trypanosoma evansi in experimental male and female mice. Onderstepoort Journal of Veterinary Research, 86(1), e1-e10.
Benaissa, M. H., Mimoune, N., Bentria, Y., Kernif, T., Boukhelkhal, A., Youngs, C. R., Kaidi, R., Faye, B., & Halis, Y. (2020). Seroprevalence and risk factors for Trypanosoma evansi, the causative agent of surra, in the dromedary camel (Camelus dromedarius) population in Southeastern Algeria. Onderstepoort Journal of Veterinary Research, 87(1), e1-e9.
Biéler, S., Matovu, E., Mitashi, P., Ssewannyana, E., Bi Shamamba, S. K., Bessell, P. R., & Ndung'u, J. M. (2012). Improved detection of Trypanosoma brucei by lysis of red blood cells, concentration and LED fluorescence microscopy. Acta Tropica, 121(2), 135–140.
Biteau, N., Asencio, C., Izotte, J., Rousseau, B., Fèvre, M., Pillay, D., & Baltz, T. (2016). Trypanosoma brucei gambiense Infections in Mice Lead to Tropism to the Reproductive Organs, and Horizontal and Vertical Transmission. PLOS Neglected Tropical Diseases, 10(1), e0004350.
Boada-Sucre, A. A., Spadafora, M. S. R., Tavares-Marques, L. M., Finol, H. J., & Reyna-Bello, A. (2016). Trypanosoma vivax Adhesion to Red Blood Cells in Experimentally Infected Sheep. Pathology Research International, 2016(1), 4503214.
Bonney, K. M., Taylor, J. M., Daniels, M. D., Epting, C. L., & Engman, D. M. (2011). Heat-killed Trypanosoma cruzi induces acute cardiac damage and polyantigenic autoimmunity. PLOS ONE, 6(1), e14571.
Bossard, G., Rodrigues, V., Tour, E., & Geiger, A. (2021). Mice immunization with Trypanosoma brucei gambiense translationally controlled tumor protein modulates immunoglobulin and cytokine production, as well as parasitaemia and mice survival after challenge with the parasite. Infection, Genetics and Evolution, 87(1), 104636.
Boushaki, D., Adel, A., Dia, M. L., Büscher, P., Madani, H., Brihoum, B. A., Sadaoui, H., Bouayed, N., & Issad, N. K. (2019). Epidemiological investigations on Trypanosoma evansi infection in dromedary camels in the South of Algeria. Heliyon, 5(7), e02086.
Bouteille, B., & Buguet, A. (2012). The detection and treatment of human African trypanosomiasis. Research and Reports in Tropical Medicine, 3(1), 35–45.
Carvalho, T., Trindade, S., Pimenta, S., Santos, A. B., Rijo-Ferreira, F., & Figueiredo, L. M. (2018). Trypanosoma brucei triggers a marked immune response in male reproductive organs. PLOS Neglected Tropical Diseases, 12(8), e0006690.
Chaplin, D. D. (2010). Overview of the immune response. Journal of Allergy and Clinical Immunology, 125(2 Suppl 2), S3–23.
Chappuis, F., Loutan, L., Simarro, P., Lejon, V., & Büscher, P. (2005). Options for field diagnosis of human african trypanosomiasis. Clinical Microbiology Reviews, 18(1), 133–146.
Chau, N. V. V., Chau, L. B., Desquesnes, M., Herder, S., Lan, N. P. H., Campbell, J. I., Cuong, N. V., Yimming, B., Chalermwong, P., Jittapalapong, S., Franco, J. R., Tue, N. T., Rabaa, M. A., Carrique-Mas, J., Thanh, T. P. T., Thieu, N. T. V., Berto, A., Hoa, N. T., Hoang, N. V. M., Tu, N. C., Chuyen, N. K., Wills, B., Hien, T. T., Thwaites, G. E., Yacoub, S., & Baker, S. (2016). A Clinical and Epidemiological Investigation of the First Reported Human Infection With the Zoonotic Parasite Trypanosoma evansi in Southeast Asia. Clinical Infectious Diseases, 62(8), 1002–1008.
Choi, B., Vu, H. T., Vu, H. T., Radwanska, M., & Magez, S. (2024). Advances in the Immunology of the Host–Parasite Interactions in African Trypanosomosis, including Single-Cell Transcriptomics. Pathogens, 13(3), 188.
Chuenkova, M. V., & Pereiraperrin, M. (2010). Trypanosoma cruzi-Derived Neurotrophic Factor: Role in Neural Repair and Neuroprotection. Journal of Neuroparasitology, 1(1), 55–60.
Claes, F., Büscher, P., Touratier, L., & Goddeeris, B. M. (2005). Trypanosoma equiperdum: master of disguise or historical mistake? Trends in Parasitology, 21(7), 316–321.
Cortes-Serra, N., Gualdron-Lopez, M., Pinazo, M. J., Torrecilhas, A. C., & Fernandez-Becerra, C. (2022). Extracellular Vesicles in Trypanosoma cruzi Infection: Immunomodulatory Effects and Future Perspectives as Potential Control Tools against Chagas Disease. Journal of Immunology Research, 2022(1), 5230603.
Costa, T. F. R., Goundry, A., Morrot, A., Grab, D. J., Mottram, J. C., & Lima, A. P. C. A. (2022). Trypanosoma brucei rhodesiense Inhibitor of Cysteine Peptidase (ICP) Is Required for Virulence in Mice and to Attenuate the Inflammatory Response. International Journal of Molecular Sciences, 24(1), 656.
Da Silva, A. S., Fanfa, V. R., Otto, M. A., Gressler, L. T., Tavares, K. C., Lazzarotto, C. R., Tonin, A. A., Miletti, L. C., Duarte, M. M., & Monteiro, S. G. (2011). Susceptibility of mice to Trypanosoma evansi treated with human plasma containing different concentrations of apolipoprotein L-1. Korean Journal of Parasitology, 49(4), 427–430.
Da Silva, A. S., Zanette, R. A., Wolkmer, P., Costa, M. M., Garcia, H. A., Lopes, S. T., Santurio, J. M., Teixeira, M. M., & Monteiro, S. G. (2009). Diminazene aceturate in the control of Trypanosoma evansi infection in cats. Veterinary Parasitology, 165(1–2), 47–50.
Dargantes, A. P., Campbell, R. S., Copeman, D. B., & Reid, S. A. (2005). Experimental Trypanosoma evansi infection in the goat. II. Pathology. Journal of Comparative Pathology, 133(4), 267–276.
Dargantes, A. P., Wardhana, A. H., Abella, J. A. C., Sequito, M. R., Reid, S. A., Copeman, D. B., & Dargantes, K. A. T. (2021). Pathogenicity of Philippine and Indonesian Trypanosoma evansi Isolates in Mice and Their Responses to Trypanocides. Jurnal Ilmu Ternak Veteriner, 26(1), 22–30.
Dario, M. A., Pavan, M. G., Rodrigues, M. S., Lisboa, C. V., Kluyber, D., Desbiez, A. L. J., Herrera, H. M., Roque, A. L. R., Lima, L., Teixeira, M. M. G., & Jansen, A. M. (2021). Trypanosoma rangeli Genetic, Mammalian Hosts, and Geographical Diversity from Five Brazilian Biomes. Pathogens, 10(6), 736.
Deleeuw, V., Phạm, H. T. T., De Poorter, I., Janssens, I., De Trez, C., Radwanska, M., & Magez, S. (2019). Trypanosoma brucei brucei causes a rapid and persistent influx of neutrophils in the spleen of infected mice. Parasite Immunology, 41(10), e12664.
Desquesnes, M., Holzmuller, P., Lai, D. H., Dargantes, A., Lun, Z. R., & Jittaplapong, S. (2013). Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Research International, 2013(1), 194176.
Desquesnes, M., Sazmand, A., Gonzatti, M., Boulangé, A., Bossard, G., Thévenon, S., Gimonneau, G., Truc, P., Herder, S., Ravel, S., Sereno, D., Waleckx, E., Jamonneau, V., Jacquiet, P., Jittapalapong, S., Berthier, D., Solano, P., & Hébert, L. (2022). Diagnosis of animal trypanosomoses: proper use of current tools and future prospects. Parasit & Vectors, 15(1), 235.
Dobson, R. J., Dargantes, A. P., Mercado, R. T., & Reid, S. A. (2009). Models for Trypanosoma evansi (surra), its control and economic impact on small-hold livestock owners in the Philippines. International Journal for Parasitology, 39(10), 1115–1123.
Dollet, M., Sturm, N. R., & Campbell, D. A. (2012). The internal transcribed spacer of ribosomal RNA genes in plant trypanosomes (Phytomonas spp.) resolves 10 groups. Infection, Genetics and Evolution, 12(2), 299–308.
Elliott, E. B., McCarroll, D., Hasumi, H., Welsh, C. E., Panissidi, A. A., Jones, N. G., Rossor, C. L., Tait, A., Smith, G. L., Mottram, J. C., Morrison, L. J., & Loughrey, C. M. (2013). Trypanosoma brucei cathepsin-L increases arrhythmogenic sarcoplasmic reticulum-mediated calcium release in rat cardiomyocytes. Cardiovascular Research, 100(2), 325–335.
Eyob, E., & Matios, L. (2013). Review on camel trypanosomosis (surra) due to Trypanosoma evansi: Epidemiology and host response. Journal of Veterinary Medicine and Animal Health, 5(12), 334–343.
Farikou, O., Simo, G., Njiokou, F., Ngassé, G. I. K., Fru, M. A., & Geiger, A. (2023). Trypanosome Infections and Anemia in Cattle Returning from Transhumance in Tsetse-Infested Areas of Cameroon. Microorganisms, 11(3), 712.
Fetene, E., Leta, S., Regassa, F., & Büscher, P. (2021). Global distribution, host range and prevalence of Trypanosoma vivax: a systematic review and meta-analysis. Parasit & Vectors, 14(1), 80.
Firdausy, L. W., Fikri, F., Wicaksono, A. P., Çalışkan, H., & Purnama, M. T. E. (2025). Prevalence of trypanosomiasis in domesticated animals in Indonesia: A systematic review and meta-analysis. Veterinary World, 18(5), 1333–1344.
Franco, J. R., Simarro, P. P., Diarra, A., & Jannin, J. G. (2014). Epidemiology of human African trypanosomiasis. Clinical Epidemiology, 6(1), 257–275.
Gao, J. M., Qian, Z. Y., Hide, G., Lai, D. H., Lun, Z. R., & Wu, Z. D. (2020). Human African trypanosomiasis: the current situation in endemic regions and the risks for non-endemic regions from imported cases. Parasitology, 147(9), 922–931.
Geiger, A., Malele, I., Abd-Alla, A. M., & Njiokou, F. (2018). Blood feeding tsetse flies as hosts and vectors of mammals-pre-adapted African Trypanosoma: current and expected research directions. BMC Microbiology, 18(Suppl 1), 162.
Getahun, M. N., Ngiela, J., Makwatta, J. O., Ahuya, P., Simon, T. K., Kamau, S. K., Torto, B., & Masiga, D. (2022). Metabolites From Trypanosome-Infected Cattle as Sensitive Biomarkers for Animal Trypanosomosis. Frontiers in Microbiology, 13(1), 922760.
Giordani, F., Morrison, L. J., Rowan, T. G., De Koning, H. P., & Barrett, M. P. (2016). The animal trypanosomiases and their chemotherapy: a review. Parasitology, 143(14), 1862–1889.
Gizaw, Y., Megersa, M., & Fayera, T. (2017). Dourine: a neglected disease of equids. Tropical Animal Health and Production, 49(5), 887–897.
Guiton, R., & Drevet, J. R. (2023). Viruses, bacteria and parasites: infection of the male genital tract and fertility. Basic and Clinical Andrology, 33(1), 19.
Hairani, B., Hadi, U. K., & Supriyono. (2023). Species diversity and daily infestation patterns of Haematophagus flies in cattle farms at Tanah Bumbu District, South Kalimantan Province, Indonesia. Biodiversitas, 24(5), 2995–3003.
Herrera, H. M., Alessi, A. C., Marques, L. C., Santana, A. E., Aquino, L. P., Menezes, R. F., Moraes, M. A., & Machado, R. Z. (2002). Experimental Trypanosoma evansi infection in South American coati (Nasua nasua): hematological, biochemical and histopathological changes. Acta Tropica, 81(3), 203–210.
Hoare, C. A. (1964). Morphological and taxonomic studies on mammalian trypanosomes. X. Revision of the systematics. The Journal of Protozoology, 11(1), 200–207.
Ilbeigi, K., Mabille, D., Roy, R., Bundschuh, M., Van de Velde, E., Hulpia, F., Van Calenbergh, S., & Caljon, G. (2025). 3'-deoxytubercidin: A potent therapeutic candidate for the treatment of Surra and Dourine. Int J Parasitol Drugs Drug Resist, 27, 100580.
Ilboudo, K., Boulangè, A., Hounyèmè, R. E., Gimonneau, G., Kaborè, J., Belem, A. G. M., Desquesnes, M., Lejon, V., Koffi, M., Jamonneau, V., & Thèvenon, S. (2023). Performance of diagnostic tests for Trypanosoma brucei brucei in experimentally infected pigs. PLOS Neglected Tropical Diseases, 17(11), e0011730.
Kamidi, C. M., Saarman, N. P., Dion, K., Mireji, P. O., Ouma, C., Murilla, G., Aksoy, S., Schnaufer, A., & Caccone, A. (2017). Multiple evolutionary origins of Trypanosoma evansi in Kenya. PLOS Neglected Tropical Diseases, 11(9), e0005895.
Kasozi, K. I., MacLeod, E. T., Ntulume, I., & Welburn, S. C. (2022). An Update on African Trypanocide Pharmaceutics and Resistance. Frontiers in Veterinary Science, 9(1), 828111.
Keatley, S., Botero, A., Fosu-Nyarko, J., Pallant, L., Northover, A., & Thompson, R. C. A. (2020). Species-level identification of trypanosomes infecting Australian wildlife by High-Resolution Melting - Real Time Quantitative Polymerase Chain Reaction (HRM-qPCR). International Journal for Parasitology: Parasites and Wildlife, 13(1), 261–268.
Kengradomkij, C., Jhaiaun, P., Chimnoi, W., Piliean, N., Inpankaew, T., & Kamyingkird, A. K. (2025). Prevalence of Trypanosoma evansi infection in Thai and imported beef cattle on the Thai-Myanmar border using parasitological and molecular methods. Veterinary World, 18(2), 500-507.
Kennedy, P. G. (2004). Human African trypanosomiasis of the CNS: current issues and challenges. Journal of Clinical Investigation, 113(4), 496–504.
Kim, J., Álvarez-Rodríguez, A., Li, Z., Radwanska, M., & Magez, S. (2023). Recent Progress in the Detection of Surra, a Neglected Disease Caused by Trypanosoma evansi with a One Health Impact in Large Parts of the Tropic and Sub-Tropic World. Microorganisms, 12(1), 44.
La Greca, F., & Magez, S. (2011). Vaccination against trypanosomiasis: can it be done or is the trypanosome truly the ultimate immune destroyer and escape artist? Human Vaccines, 7(11), 1225–1233.
Langousis, G., & Hill, K. L. (2014). Motility and more: the flagellum of Trypanosoma brucei. Nature Reviews Microbiology, 12(7), 505–518.
Latif, A. A., Ntantiso, L., & de Beer, C. (2019). African animal trypanosomosis (nagana) in northern KwaZulu-Natal, South Africa: Strategic treatment of cattle on a farm in endemic area. Onderstepoort Journal of Veterinary Research, 86(1), a1639.
Lendzele, S. S., Abah, S., Nguetoum, C., Burinyuy, K. A., Koumba, A. A., & Mavoungou, J. F. (2022). Tabanid-transmitted animal trypanosomiasis in Cameroon: Evidence from a study in the tsetse free pastoral zone of Galim. Parasite Epidemiology and Control, 18(1), e00253.
Lewis, M. D., Francisco, A. F., Taylor, M. C., & Kelly, J. M. (2015). A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. Journal of Biomolecular Screening, 20(1), 36–43.
Lindner, A. K. & Priotto, G. (2010). The unknown risk of vertical transmission in sleeping sickness--a literature review. PLOS Neglected Tropical Diseases, 4(12), e783.
Lobo, M., Balouz, V., Melli, L., Carlevaro, G., Cortina, M. E., Cámara, M. L. M., Cánepa, G. E., Carmona, S. J., Altcheh, J., Campetella, O., Ciocchini, A. E., Agüero, F., Mucci, J., & Buscaglia, C. A. (2019). Molecular and antigenic characterization of Trypanosoma cruzi TolT proteins. PLOS Neglected Tropical Diseases, 13(3), e0007245.
Lumbala, C., Biéler, S., Kayembe, S., Makabuza, J., Ongarello, S., & Ndung'u, J. M. (2018). Prospective evaluation of a rapid diagnostic test for Trypanosoma brucei gambiense infection developed using recombinant antigens. PLOS Neglected Tropical Diseases, 12(3), e0006386.
MacLean, L., Reiber, H., Kennedy, P. G., & Sternberg, J. M. (2012). Stage progression and neurological symptoms in Trypanosoma brucei rhodesiense sleeping sickness: role of the CNS inflammatory response. PLOS Neglected Tropical Diseases, 6(10), e1857.
Magez, S., Schwegmann, A., Atkinson, R., Claes, F., Drennan, M., De Baetselier, P., & Brombacher, F. (2008). The role of B-cells and IgM antibodies in parasitemia, anemia, and VSG switching in Trypanosoma brucei-infected mice. PLOS Pathogens, 4(8), e1000122.
Magez, S., Torres, J. E. P., Obishakin, E., & Radwanska, M. (2020). Infections With Extracellular Trypanosomes Require Control by Efficient Innate Immune Mechanisms and Can Result in the Destruction of the Mammalian Humoral Immune System. Frontiers in Immunology, 11(1), 382.
Magri, A., Galuppi, R., & Fioravanti, M. (2021). Autochthonous Trypanosoma spp. in European Mammals: A Brief Journey amongst the Neglected Trypanosomes. Pathogens, 10(3), 334.
Malafaia, G., & Talvani, A. (2011). Nutritional Status Driving Infection by Trypanosoma cruzi: Lessons from Experimental Animals. Journal of Tropical Medicine, 2011(1), 981879.
Manuel, M. F. (1998). Sporadic outbreaks of Surra in the Philippines. Journal of Protozoology Research, 8(3), 131–138.
Martín-Escolano, J., Marín, C., Rosales, M. J., Tsaousis, A. D., Medina-Carmona, E., & Martín-Escolano, R. (2022). An Updated View of the Trypanosoma cruzi Life Cycle: Intervention Points for an Effective Treatment. ACS Infectious Diseases, 8(6), 1107–1115.
Matthews, K.R. (2005). The developmental cell biology of Trypanosoma brucei. Journal of Cell Science, 118(Pt 2), 283–290.
Mdachi, R. E., Ogolla, K. O., Auma, J. E., Wamwiri, F. N., Kurgat, R. K., Wanjala, K. B., Mugunieri, L. G., Alusi, P. M., Chemuliti, J. K., Mukiria, P. W., & Okoth, S. O. (2023). Variation of sensitivity of Trypanosoma evansi isolates from Isiolo and Marsabit counties of Kenya to locally available trypanocidal drugs. PLOS ONE, 18(2), e0281180.
Mihok, S. (2002). The development of a multipurpose trap (the Nzi) for tsetse and other biting flies. Bulletin of Entomological Research, 92(5), 385–403.
Misra, K. K., Roy, S., & Choudhury, A. (2016). Biology of Trypanosoma (Trypanozoon) evansi in experimental heterologous mammalian hosts. Journal of Parasitic Diseases, 40(3), 1047–1061.
Mogk, S., Boßelmann, C. M., Mudogo, C. N., Stein, J., Wolburg, H., & Duszenko, M. (2017). African trypanosomes and brain infection - the unsolved question. Biological reviews of the Cambridge Philosophical Society, 92(3), 1675–1687.
Morais, M. C. C., Silva, D., Milagre, M. M., de Oliveira, M. T., Pereira, T., Silva, J. S., Costa, L. D. F., Minoprio, P., Junior, R. M. C., Gazzinelli, R., de Lana, M., & Nakaya, H.I. (2022). Automatic detection of the parasite Trypanosoma cruzi in blood smears using a machine learning approach applied to mobile phone images. PeerJ, 10(1), e13470.
Moreno, C. J. G., Torres, T., & Silva, M. S. (2019). Variable Surface Glycoprotein from Trypanosoma brucei Undergoes Cleavage by Matrix Metalloproteinases: An in silico Approach. Pathogens, 8(4), 178.
Morrison, L. J., Steketee, P. C., Tettey, M. D., & Matthews, K. R. (2023). Pathogenicity and virulence of African trypanosomes: From laboratory models to clinically relevant hosts. Virulence, 14(1), 2150445.
Mudji, J., Blum, A., Grize, L., Wampfler, R., Ruf, M. T., Cnops, L., Nickel, B., Burri, C., & Blum, J. (2020). Gambiense Human African Trypanosomiasis Sequelae after Treatment: A Follow-Up Study 12 Years after Treatment. Tropical Medicine and Infectious Disease, 5(1), 10.
Munir, F., Sindhu, Z. U. D., Tayyeb, M., Abbas, R. Z., Shakoor, A., Khan, A. M. A., Asrar, R., Shrafat, H., Qamar, M. H., Ahmad, S., Kauser M., & Aleem, M. T. (2023). Rationale to Develop mRNA-based Vaccines for Trypanosoma brucei (a review). Continental Veterinary Journal, 3(1), 26–35.
Nguyen, H. T. T., Guevarra, R. B., Magez, S., & Radwanska, M. (2021). Single-cell transcriptome profiling and the use of AID deficient mice reveal that B cell activation combined with antibody class switch recombination and somatic hypermutation do not benefit the control of experimental trypanosomosis. PLOS Pathogens, 17(11), e1010026.
Njiru, Z. K., Ndung'u, K., Matete, G., Ndungu, J. M., & Gibson, W. C. (2004). Detection of Trypanosoma brucei rhodesiense in animals from sleeping sickness foci in East Africa using the serum resistance associated (SRA) gene. Acta Tropica, 90(3), 249–254.
Nuryady, M. M., Widayanti, R., Nurcahyo, R. W., Fadjrinatha, B., & Fahrurrozi, Z. S. A. (2019). Characterization and phylogenetic analysis of multidrug-resistant protein - encoding genes in Trypanosoma evansi isolated from buffaloes in Ngawi district, Indonesia. Veterinary World, 12(10), 1573–1577.
Okello, I., Nzalawahe, J., Mafie, E., & Eastwood, G. (2023). Seasonal variation in tsetse fly apparent density and Trypanosoma spp. infection rate and occurrence of drug-resistant trypanosomes in Lambwe, Kenya. Parasitology Research, 123(1), 46.
Okello, W. O., MacLeod, E. T., Muhanguzi, D., Waiswa, C., & Welburn, S. C. (2021). Controlling Tsetse Flies and Ticks Using Insecticide Treatment of Cattle in Tororo District Uganda: Cost Benefit Analysis. Frontiers in Veterinary Science, 8(1), 616865.
Pathak, A. K. (2009). Effect of Trypanosoma spp. on Nutritional status and performance of livestock. Veterinary World, 2(11), 435–438.
Pereira, S. S., Trindade, S., De Niz, M., & Figueiredo, L. M. (2019). Tissue tropism in parasitic diseases. Open Biology, 9(5), 190036.
Phongphaew, W., Wongsali, C., Boonyakong, T., Samritwatchasai, T., Chimnoi, W., & Kamyingkird, K. (2023). Histopathology and virulence of an in vitro-adapted Trypanosoma evansi TEDC 953 strain (Thailand isolate) in mice. Veterinary World, 16(5), 1008–1017.
Ponte-Sucre, A. (2016). An Overview of Trypanosoma brucei Infections: An Intense Host-Parasite Interaction. Frontiers in Microbiology, 7(1), 2126.
Pruvot, M., Kamyingkird, K., Desquesnes, M., Sarataphan, N., & Jittapalapong, S. (2010). A comparison of six primer sets for detection of Trypanosoma evansi by polymerase chain reaction in rodents and Thai livestock. Veterinary Parasitology, 171(3–4), 185–193.
Quiroga, N., Campos-Soto, R., Yañez-Meza, A., Pedro, A. R. S., Allendes, J. L., Bacigalupo, A., Botto-Mahan, C., & Correa, J. P. (2022). Trypanosoma cruzi DNA in Desmodus rotundus (common vampire bat) and Histiotus montanus (small big-eared brown bat) from Chile. Acta Tropica, 225(1), 106206.
Radwanska, M., Vereecke, N., Deleeuw, V., Pinto, J., & Magez, S. (2018). Salivarian Trypanosomosis: A Review of Parasites Involved, Their Global Distribution and Their Interaction With the Innate and Adaptive Mammalian Host Immune System. Frontiers in Immunology, 9(1), 2253.
Reck, C., Menin, Á., Batista, F., Santos, P. O. M., & Miletti, L. C. (2021). Evaluation of buffered Trypanosoma evansi antigen and rapid serum agglutination test (BA/Te) for the detection of anti-T. evansi antibodies in horses in Brazil. Curr. Res. Parasitol. Vector-Borne Diseases, 1(1), 100024.
Roy, N., Nageshan, R. K., Pallavi, R., Chakravarthy, H., Chandran, S., Kumar, R., Gupta, A. K., Singh, R. K., Yadav, S. C., & Tatu, U. (2010). Proteomics of Trypanosoma evansi infection in rodents. PLOS ONE, 5(3), e9796.
Saldanha, I., Betson, M., Vrettou, C., Paxton, E., Nixon, J., Tennant, P., Ritchie, A., Matthews, K. R., Morrison, L. J., Torr, S. J., & Cunningham, L. J. (2024). Consistent detection of Trypanosoma brucei but not T. congolense DNA in faeces of experimentally infected cattle. Scientific Reports, 14(1), 4158.
Sari, F. R., Fahrimal, Y., Balqis, U., Subekti, D. T., Wardana, A., & Hambal, M. (2015). Parasitemia Level of Male White Mice (Mus musculus L.) DDY Strain Infected with Trypanosoma evansi of Pidie and Pemalang Isolate. Jurnal Medik Veteriner, 8(2), 85–87.
Sawitri, D. H., Wardhana, A. H., Sadikin, M., & Wibowo, H. (2019). Detection of Surra (trypanosomiasis) positivity in humans in an outbreak area of Indonesia. Medical Journal of Indonesia, 28(2), 196–202.
Sazmand, A., Joachim, A., & Otranto, D. (2019). Zoonotic parasites of dromedary camels: so important, so ignored. Parasites & Vectors, 12(1), 610.
Schuster, S., Krüger, T., Subota, I., Thusek, S., Rotureau, B., Beilhack, A., & Engstler, M. (2017). Developmental adaptations of trypanosome motility to the tsetse fly host environments unravel a multifaceted in vivo microswimmer system. Elife, 6(1), e27656.
Schuster, S., Lisack, J., Subota, I., Zimmermann, H., Reuter, C., Mueller, T., Morriswood, B. & Engstler, M. (2021). Unexpected plasticity in the life cycle of Trypanosoma brucei. Elife, 10(1), e66028.
Selim, A., Alafari, H. A., Attia, K., AlKahtani, M. D. F., Albohairy, F. M., & Elsohaby, I. (2022). Prevalence and animal level risk factors associated with Trypanosoma evansi infection in dromedary camels. Scientific Reports, 12(1), 8933.
Setiawan, A., Nurcahyo, W., Priyowidodo, D., Budiati, R. T., & Susanti, D. S. R. (2021). Genetic and parasitological identification of Trypanosoma evansi infecting cattle in South Sulawesi, Indonesia. Veterinary World, 14(1), 113–119.
Silva, R. A., Arosemena, N. A., Herrera, H. M., Sahib, C. A., & Ferreira, M. S. (1995). Outbreak of trypanosomosis due to Trypanosoma evansi in horses of Pantanal Mato-grossense, Brazil. Veterinary Parasitology, 60(1–2), 167–171.
Silvester, E., McWilliam, K. R., & Matthews, K. R. (2017a). The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma brucei in the Mammalian Bloodstream. Pathogens, 6(3), 29.
Silvester, E., Young, J., Ivens, A., & Matthews, K. R. (2017b). Interspecies quorum sensing in co-infections can manipulate trypanosome transmission potential. Nature Microbiology, 2(1), 1471–1479.
Sivajothi, S., & Reddy, B. S. (2019). Rare report of orchitis in a bull due to Trypanosoma evansi infection. Journal of Parasitic Diseases, 43(1), 28–30.
Snijders, R., Fukinsia, A., Claeys, Y., Hasker, E., Mpanya, A., Miaka, E., Meheus, F., & Boelaert, M. (2021). Costs and Outcomes of Integrated Human African Trypanosomiasis Surveillance System Using Rapid Diagnostic Tests, Democratic Republic of the Congo. Emerging Infectious Diseases, 27(8), 2144–2153.
Soeters, P. B., Wolfe, R. R., & Shenkin, A. (2019). Hypoalbuminemia: Pathogenesis and Clinical Significance. Journal of Parenteral and Enteral Nutrition, 43(2), 181–193.
Somoza, M., Bertelli, A., Pratto, C. A., Verdun, R. E., Campetella, O., & Mucci, J. (2022). Trypanosoma cruzi Induces B Cells That Regulate the CD4+ T Cell Response. Frontiers in Cellular and Infection Microbiology, 11(1), 789373.
Steverding, D. (2008). The history of African trypanosomiasis. Parasit & Vectors, 1(1), 3.
Stijlemans, B., Caljon, G., Van Den Abbeele, J., Van Ginderachter, J. A., Magez, S., & De Trez, C. (2016). Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Frontiers in Immunology, 7(1), 233.
Stijlemans, B., De Baetselier, P., Magez, S., Van Ginderachter, J. A., & De Trez, C. (2018). African Trypanosomiasis-Associated Anemia: The Contribution of the Interplay between Parasites and the Mononuclear Phagocyte System. Frontiers in Immunology, 9(1), 218.
Stijlemans, B., Schoovaerts, M., De Baetselier, P., Magez, S., & De Trez, C. (2022). The Role of MIF and IL-10 as Molecular Yin-Yang in the Modulation of the Host Immune Microenvironment During Infections: African Trypanosome Infections as a Paradigm. Frontiers in Immunology, 13(1), 865395.
Su, B. X., Wang, J. F., Yang, T. B., Hide, G., Lai, D. H., & Lun, Z. R. (2022). A new species of mammalian trypanosome, Trypanosoma (Megatrypanum) bubalisi sp. nov., found in the freshwater leech Hirudinaria manillensis. International Journal for Parasitology, 52(4), 253–264.
Suganuma, K., Narantsatsral, S., Battur, B., Yamasaki, S., Otgonsuren, D., Musinguzi, S. P., Davaasuren, B., Battsetseg, B., & Inoue, N. (2016). Isolation, cultivation and molecular characterization of a new Trypanosoma equiperdum strain in Mongolia. Parasites & Vectors, 9(1), 481.
Suprihati, E., Suwanti, L. T., Yudhana, A., & Kusumaningrum, A. I. (2022). Comparison of ITS-1 and TBR-1/2 primer sensitivity for the detection of Trypanosoma evansi local isolates in experimental rats using a polymerase chain reaction. Veterinary World, 15(7), 1772–1778.
Tamarit, A., Gutierrez, C., Arroyo, R., Jimenez, V., Zagalá, G., Bosch, I., Sirvent, J., Alberola, J., Alonso, I., & Caballero, C. (2010). Trypanosoma evansi infection in mainland Spain. Veterinary Parasitology, 167(1), 74–76.
Tao, J., Kamanaka, M., Hao, J., Hao, Z., Jiang, X., Craft, J. E., Flavell, R. A., Wu, Z., Hong, Z., Zhao, L., & Yin, Z. (2011). IL-10 signaling in CD4+ T cells is critical for the pathogenesis of collagen-induced arthritis. Arthritis Research & Therapy, 13(6), R212.
Tejero, F., Roschman-González, A., Perrone-Carmona, T. M., & Aso, P. M. (2008). Trypanosoma evansi: A quantitative approach to the understanding of the morphometry-hematology relationship throughout experimental murine infections. The Journal of Protozoology Research, 18(1), 34–47.
Telleria, E. L., Benoit, J. B., Zhao, X., Savage, A. F., Regmi, S., Silva, T. L. A., O'Neill, M., & Aksoy, S. (2014). Insights into the trypanosome-host interactions revealed through transcriptomic analysis of parasitized tsetse fly salivary glands. PLOS Neglected Tropical Diseases, 8(4), e2649.
Thuita, J. K., Kagira, J. M., Mwangangi, D., Matovu, E., Turner, C. M., & Masiga, D. (2008). Trypanosoma brucei rhodesiense transmitted by a single tsetse fly bite in vervet monkeys as a model of human African trypanosomiasis. PLOS Neglected Tropical Diseases, 2(5), e238.
Tran, T., Claes, F., Verloo, D., De Greve, H., & Büscher, P. (2009). Towards a new reference test for surra in camels. Clinical and Vaccine Immunology, 16(7), 999–1002.
Van Den Abbeele, J., Caljon, G., De Ridder, K., De Baetselier, P., & Coosemans, M. (2010). Trypanosoma brucei modifies the tsetse salivary composition, altering the fly feeding behavior that favors parasite transmission. PLoS Pathogens, 6(6), e1000926.
Venturelli, A., Tagliazucchi, L., Lima, C., Venuti, F., Malpezzi, G., Magoulas, G. E., Santarem, N., Calogeropoulou, T., Cordeiro-da-Silva, A., & Costi, M. P. (2022). Current Treatments to Control African Trypanosomiasis and One Health Perspective. Microorganisms, 10(7), 1298.
von Wissmann, B., Machila, N., Picozzi, K., Fèvre, E. M., deC Bronsvoort, B. M., Handel, I. G., & Welburn, S. C. (2011). Factors associated with acquisition of human infective and animal infective trypanosome infections in domestic livestock in Western Kenya. PLOS Neglected Tropical Diseases, 5(1), e941.
Wei, R., Li, X., Wang, X., Zhang, N., Wang, Y., Zhang, X., Gong, P., & Li, J. (2021). Trypanosoma evansi evades host innate immunity by releasing extracellular vesicles to activate TLR2-AKT signaling pathway. Virulence, 12(1), 2017–2036.
Wheeler, R. J., Gull, K., & Sunter, J. D. (2019). Coordination of the Cell Cycle in Trypanosomes. Annual Review of Microbiology, 73(1), 133–154.
Williams, J. T., Mubiru, J. N., Schlabritz-Loutsevitch, N. E., Rubicz, R. C., VandeBerg, J. L., Dick, E. J. Jr., & Hubbard, G. B. (2009). Polymerase chain reaction detection of Trypanosoma cruzi in Macaca fascicularis using archived tissues. American Journal of Tropical Medicine and Hygiene, 81(2), 228–234.
Witola, W. H., Sarataphan, N., Inoue, N., Ohashi, K., & Onuma, M. (2005). Genetic variability in ESAG6 genes among Trypanosoma evansi isolates and in comparison to other Trypanozoon members. Acta Tropica, 93(1), 63–73.
Yasine, A., Ashenafi, H., Geldhof, P., Van Brantegem, L., Vercauteren, G., Bekana, M., Tola, A., Van Soom, A., Duchateau, L., Goddeeris, B., & Govaere, J. (2019). Histopathological lesions in reproductive organs, distal spinal cord and peripheral nerves of horses naturally infected with Trypanosoma equiperdum. BMC Veterinary Research, 15(1), 175.
Young, R., Taylor, J. E., Kurioka, A., Becker, M., Louis, E. J., & Rudenko, G. (2008). Isolation and analysis of the genetic diversity of repertoires of VSG expression site containing telomeres from Trypanosoma brucei gambiense, T. b. brucei, and T. equiperdum. BMC Genomics, 9(1), 385.
Copyright (c) 2025 Sunaryo Hadi Warsito, Aswin Rafif Khairullah, Mirni Lamid, Mohammad Anam Al-Arif, Herry Agoes Hermadi, Widya Paramita Lokapirnasari, Muhammad Khaliim Jati Kusala, Syahputra Wibowo, Siti Rani Ayuti, Bantari Wisynu Kusuma Wardhani, Ima Fauziah, Sheila Marty Yanestria, Ikechukwu Benjamin Moses, Agung Prasetyo, Suhita Aryaloka, Kartika Afrida Fauzia, Riza Zainuddin Ahmad, Dea Anita Ariani Kurniasih

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish in this journal agree to the following terms:
1. The journal allows the author to hold the copyright of the article without restrictions;
2. The journal allows the author(s) to retain publishing rights without restrictions;
3. The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA).






11.jpg)