OPTIMIZATION OF ANNEALING TEMPERATURE AND PRIMER CONCENTRATION OF CYTOCHROME B (CYT B) GENE FOR PIG DNA DETECTION WITH REAL-TIME PCR METHOD
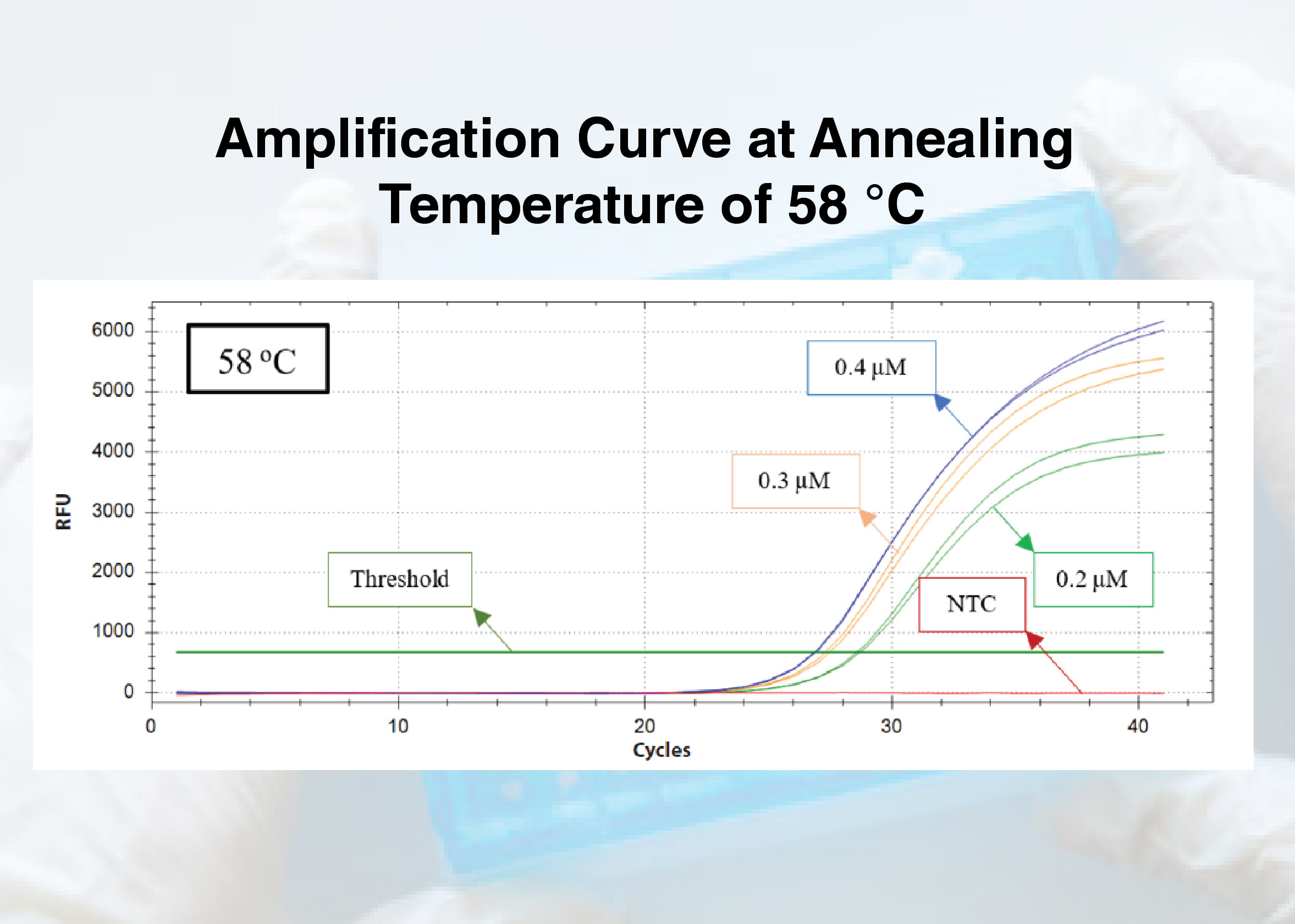
Background: One company in Indonesia has developed a pig DNA detection kit by designing primers with the real-time Polymerase Chain Reaction (PCR) method using the cytochrome b (cyt b) gene. It is necessary to optimize the PCR process to optimize pig DNA detection, including annealing temperature and primer concentration, which can increase sensitivity, specificity, and precision. Purpose: This research aims to determine the optimum annealing temperature and primer concentration for the detection of pig DNA using cyt b gene. Method: In this research, the extracted sample isolates were subjected to 12 treatments with 2 repetitions. Optimal data analysis was based on the lowest Cycle Threshold (CT) value in the amplification curve. Result: Out of a total of 24 samples, an increase in the CT value was observed at annealing temperatures of 57 °C, 59 °C, and 60 °C compared to 58 °C, across various primer concentrations. The primer concentrations with the lowest CT values were successively found to be 0.4 μM, 0.3 μM, and 0.2 μM. Conclusion: The results of the research that has been conducted indicate that the optimal annealing temperature for detecting pig DNA using the cyt b gene in this research is 58 °C, and the optimal concentrations of forward and reverse primers are 0.4 μM.
Introduction
Heat-cured acrylic resin is the most commonly used denture base material. Heat-cured acrylic resin, known as acrylic resin, has experienced thermal activation polymerization (Anjai et al., 2018(Anjai et al., 2018)). This material has a gingiva like texture and color, making it aesthetically pleasing, affordable, and easy to manipulate and repair. However, heat-cured acrylic resin is also porous and tends to absorb fluid (Naini, 2015(Naini, 2015)).
Denture bases in contact with saliva formAcquired Dental Pellicle(ADP) and generate plaque formation, which increases the accumulation of microorganisms in the oral cavity, especiallyCandida albicans. This condition results in denture stomatitis (Dama, 2013(Dama, 2013); Wirayuni, 2017(Wirayuni, 2017)). Periodic cleaning using a denture cleanser is essential to prevent this possibility. However, chemical denture cleansers are reported to increase the surface roughness of heat-cured acrylic resin due to its acid content. Studies suggest using natural or herbal ingredients to minimize this drawback (Dewi et al., 2020(Dewi et al., 2020)).
Edamame (Glycine max L. Merril) is a soybean from the legume group, harvested at the peak of ripening before reaching the hardening period. This plant is originally from Japan. Edamame contains essential compounds such as flavonoids, saponins, isoflavones, alkaloids, and tannins, which have antifungal and antibacterial properties (Abd-Alla et al., 2019(Abd-Alla et al., 2019); Abdel-Hadya et al., 2019(Abdel-Hadya et al., 2019)). A previous study proved that edamame extracts at a concentration of 50 mg/ml could inhibit the growth ofCandida albicans(Igboabuchi and Ilodibia, 2018(Igboabuchi & Ilodibia, 2018); Kristiana et al., 2022(Kristiana et al., 2022)). In addition, other studies also show that 50% of edamame extracts effectively inhibit the number of Streptococcus mutans colonies (Devi et al., 2023(Devi et al., 2023)). However, flavonoid compounds in edamame in contact with heat-cured acrylic resin might cause an increase in the surface roughness of heat-cured acrylic resin.
Surface roughness is crucial for dentures since it affects their quality and longevity and induces bacteria adhesion. The ideal cleanser should keep the mechanical and physical characteristics of the denture base resin the same after extended usage (Porwal et al., 2017(Porwal et al., 2017); Sharma et al., 2017(Sharma et al., 2017)). For reference, the surface roughness of a heat-cured acrylic resin denture base is acceptable if the value is not more than 0.2 μm (Onwubu and Mdluli, 2022(Onwubu & Mdluli, 2022); Vinagre et al., 2023(Vinagre et al., 2023)).
The surface roughness of heat-cured acrylic resin dentures generally increases as dimensional changes because of the duration of use (Arafa, 2016(Arafa, 2016)). Those changes might happen during intraoral use. Ningsih et al. (2020)(Ningsih et al., 2020)suggested that after 1 to 3 years of wear. If adjusted to the contact time between the edamame extract and the acrylic resin plate for 15 minutes in short-term soaking), the usage period of one year is equivalent to four days, and three years is equal to a soaking time of 11 days (Arruda et al., 2015(Arruda et al., 2015)). Thus, we chose those periods to analyze the possibility of 50% of edamame extracts changing surface roughness on prolonged use and whether it is still clinically tolerable.
Material and Method
Specimen preparation
Twenty four heat-cured acrylic resin specimens were fabricated according to the manufacturer's guidelines using conventional flasking and pressure-pack techniques. The acrylic resin was placed in a square-shaped cuvette with a size of 10 x 10 x 2 mm (based on the American Dental Association (ADA) specification No. 12). The polymerization process of the resin was carried out by placing the flask in boiling water at 100oC for 20 minutes. The cuvette was left until it reached room temperature before opening. Subsequently, the acrylic sample was smoothed with 500 number scouring paper until the surface was flat and smooth. Then, the acrylic resin specimens were immersed in distilled water for 48 hours and in artificial saliva for one hour.
Preparation of edamame bean extract
The variant of edamame that we used in this research is MF 116 (PT Mitratani Dua Tujuh, Jember, Indonesia). First, edamame beans were dried in an oven at 50oC for one day. The dried edamame seeds were then ground into powder and sieved. Subsequently, maceration was carried out using 70% ethanol solvent at a 1 : 5 (gr/ml) ratio for three days. The obtained filtrate was then evaporated using a rotary evaporator (Siddiq, 2016(Siddiq, 2016)). Finally, 50% edamame bean extract was obtained by diluting 1.5 ml extract in 1.5 ml distilled water.
Immersion process
Twenty-four specimens of acrylic resin (n = 6/group) were distributed into two control groups and two treatment groups, namely A1 and A2 (immersion in distilled water for four days and 11 days, respectively), and B1 and B2 (immersion in 50% edamame bean extract for four and 11 days, respectively). Duration of four and 11 days for immersion was equivalent to using a denture cleanser solution for one year and three years, respectively.
The measurement of surface roughness (Ra)
The surface roughness of the samples before and after treatment was measured using a surface roughness tester TR 220. Measurements are taken on one side of the sample on three different lines. Each sample's average surface roughness (Ra) was then noted and analyzed.
Statistical analysis
The average
Amaral, J.S., Santos, G., Oliveira, M.B.P.P., Mafra, I., 2017. Quantitative Detection of Pork Meat by Evagreen Real-Time PCR to Assess The Authenticity of Processed Meat Products. Food Control Vol. 72, Pp. 53–61.
Arya, M., Shergill, I.S., Williamson, M., Gommersall, L., Arya, N., Patel, H.R.H., 2005. Basic Principles of Real-Time Quantitative PCR. Expert Review of Molecular Diagnostics Vol. 5(2), Pp. 209-219.
BIO-RAD Laboratories, 2006. Real-Time PCR Applications Guide.
BIO-RAD Laboratories, 2022. CFX96 Touch Real-Time PCR Detection System | Bio-Rad. BIO-RAD. URL https://www.bio-rad.com/en-id/product/cfx96-touch-real-time-pcr-detection-system?ID=LJB1YU15 (accessed 2.1.24).
Borah, P., 2011. Primer Designing for PCR. Science Vision Vol. 11(3), Pp. 134-136.
Bustin, S.A., Benes, V., Garson, J.A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M.W., Shipley, G.L., Vandesompele, J., Wittwer, C.T., 2009. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clinical Chemistry Vol. 55(4), Pp. 611-622.
Caraguel, C.G.B., Stryhn, H., Gagné, N., Dohoo, I.R., Hammell, K.L., 2011. Selection of A Cut Off Value for Real-Time Polymerase Chain Reaction Results to Fit A Diagnostic Purpose: Analytical and Epidemiologic Approaches. Journal of Veterinary Diagnostic Investigation: Official Publication of The American Association of Veterinary Laboratory Diagnosticians, Inc Vol. 23(1), Pp. 2-15.
Dooley, J.J., Paine, K.E., Garrett, S.D., Brown, H.M., 2004. Detection of Meat Species using Taqman Real-Time PCR Assays. Meat Science Vol. 68(3), Pp. 431-438.
Farias, I.P., Ortí, G., Sampaio, I., Schneider, H., Meyer, A., 2001. The Cytochrome b Gene as A Phylogenetic Marker: The Limits of Resolution for Analyzing Relationships among Cichlid Fishes. Journal of Molecular Evolution. Journal of Molecular Evolution Vol. 53(2), Pp. 89-103.
Gunson, R., Gillespie, G., F Carman, W., 2003. Optimisation of PCR Reactions using Primer Chessboarding. Journal of Clinical Virology Vol. 26(3), Pp. 369-373.
Handoyo, D., Rudiretna, A., 2000. Prinsip Umum dan Pelaksanaan Polymerase Chain Reaction (PCR). UNITAS Vol. 9(1), Pp. 17-29.
Harshitha, R., Arunraj, D.R., 2021. Real‐Time Quantitative PCR : A Tool for Absolute and Relative Quantification. Biochemistry and Molecular Biology Education. Biochemistry and Molecular Biology Education: A Bimonthly Publication of the International Union of Biochemistry and Molecular Biology Vol. 49(5), Pp. 800-812.
Hilscher, C., Vahrson, W., Dittmer, D.P., 2005. Faster Quantitative Real-Time PCR Protocols May Lose Sensitivity and Ahow Increased Variability. Nucleic Acids Research Vol. 33(21), Pp. e182.
Hsu, J.T., Das, S., Mohapatra, S., 1997. Polymerase Chain Reaction Engineering. Biotechnology and Bioengineering Vol. 55(2), Pp. 359-366.
Hudson, 2008. Hudson Genomics. Hudson Institute of Medical Research. URL https://www.hudson.org.au/facilities/hudson-genomics/ (accessed 2.1.24).
IDT, 2022. Primer Design Tools for PCR & Amp; qPCR. Integrated DNA Technologies. URL https://sg.idtdna.com/pages/education/decoded/article/design-efficient-pcr-and-qpcr-primers-and-probes-using-online-tools (accessed 2.1.24).
Janudin, A., Chin, N., Ahmed, M., 2022. An Eva Green Real-Time PCR Assay for Porcine DNA Analysis in Raw and Processed Foods. Malaysian Journal of Halal Research Vol. 5(1), Pp. 33039.
Joko, T., Kusumandari, N., Hartono, S., 2011. Optimasi Metode PCR untuk Deteksi Pectobacterium carotovorum, Penyebab Penyakit Busuk Lunak Anggrek. Jurnal Perlindungan Tanaman Indonesia Vol. 17(2), Pp. 54-59.
Kim, Y.S., Yu, H.K., Lee, B.Z., Hong, K.W., 2018. Effect of DNA Extraction Methods on The Detection of Porcine Ingredients in Halal Cosmetics using Real-Time PCR. Applied Biological Chemistry Vol. 61(5), Pp. 549-555.
Life Technologies, 2014. Real-Time PCR Handbook.
Markoulatos, P., Siafakas, N., Moncany, M., 2002. Multiplex Polymerase Chain Reaction: A Practical Approach. Journal of Clinical Laboratory Analysis Vol. 16(1), Pp. 47-51.
Ministry of Religion, 2014. Jaminan Produk Halal.
Pestana, E.A., Belák, S., Diallo, A., Crowther, J., Viljoen, G., 2010. Early, Rapid and Sensitive Veterinary Molecular Diagnostics-Real Time PCR Applications. Springer, Dordrecht Heidelberg, London.
Republika, 2021. Pengajuan Sertifikasi Halal Meningkat, Ini Alasannya.
Roux, K.H., 2009. Optimizing and Troubleshooting in PCR. Cold Spring Harbor Protocols Vol. 2009(4), Pp. pdbip66.
Tania, Nolan, Bergkvist, A., Carvallo, C., Chereson, P.H., Daley, L., Heath, A., Hibb, S., Hoge, S., Jouravlena, E., Kreader, C., Mohammed, M., Mueller, E., Richardson, G., Russell, T., Ward, B., Weber, S.A., Wiklander, M., 2014. A Technical Guide PCR Technologies. SIGMA-AL
Copyright (c) 2025 Journal of Vocational Health Studies

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
- The authors agree to transfer the transfer copyright of the article to the Journal of Vocational Health Studies (JVHS) effective if and when the paper is accepted for publication.
- Legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike (CC BY-NC-SA), implies that publication can be used for non-commercial purposes in its original form.
- Every publications (printed/electronic) are open access for educational purposes, research, and library. Other that the aims mentioned above, editorial board is not responsible for copyright violation.
Journal of Vocational Health Studies is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License