A CASE REPORT OF SUCCESSFUL STEROID TREATMENT IN INFANT WITH EXTRAHEPATIC CHOLESTASIS
Background: Cholestatic jaundice in infants is a significant healthcare challenge, particularly in regions where access to surgical intervention and liver transplantation is limited. An immunologic mechanism underlies the pathogenesis of biliary atresia leading to fibro-obliteration of the bile ducts. However, the successful management of biliary atresia is often difficult because treatment typically occurs at an advanced stage. Therefore, alternative therapies that can suppress bile duct inflammation are urgently needed. Administering anti-inflammatory drugs such as methylprednisolone to infants in the early stages of cholestasis may provide opportunities to improve outcomes in the limited capacity to perform Kasai surgery and liver transplantation. Purpose: This case report describes the clinical improvement of extrahepatic cholestasis following steroid administration. Case analysis: We report a case of a 24-day-old male infant presenting with clinical symptoms of jaundice and pale stool. Liver biopsy revealed features consistent with extrahepatic obstructive cholestasis characteristic of biliary atresia. The patient was treated with methylprednisolone (a corticosteroid) and ursodeoxycholic acid without surgical intervention. Result: The combination of methylprednisolone and ursodeoxycholic acid normalized liver function tests and led to significant clinical improvement. Both jaundice and pale stools completely resolved within two months of treatment. Conclusion: Steroid therapy may provide clinical benefits for infants with extrahepatic cholestasis, particularly in settings with limited healthcare resources. Steroid administration may play a role in the suppression of the inflammatory process that causes fibrosis and bile duct obliteration in the early stages of the disease.
Introduction
Cholestatic jaundice in infants remains a challenging health problem. It is often misdiagnosed as physiological jaundice(Lykavieris et al., 2005);(Saito et al., 2011). Biliary atresia can cause elevated direct bilirubin shortly after birth(Götze et al., 2015). Cholestatic jaundice needs to be treated immediately because of the risk of liver damage(Okajima et al., 2016). Therefore, any prolonged jaundice that occurs in an infant for more than 2 weeks after birth should be evaluated for cholestatic jaundice because of the risk of progressive liver damage(Feldman & Sokol, 2021);(Harpavat et al., 2011).
In confirmed cases of cholestasis, evaluation and specific therapy for suppression of liver fibrosis due to bile flow obstruction should be initiated immediately. Measuring direct and total bilirubin fractions is highly recommended for prolonged jaundice in infants(Fawaz et al., 2017). Cholestasis is typically diagnosed when direct bilirubin exceeds 1.0 mg/dL with total bilirubin below 5.0 mg/dL, or when direct bilirubin constitutes more than 20% of total bilirubin if the total level exceeds 5.0 mg/dL. Direct bilirubin levels ≥1.0 mg/dL have been shown to be more sensitive for detecting biliary atresia than the direct: total bilirubin ratio in infants aged 3 to 60 days(Lakshminarayanan & Davenport, 2016). In addition to an elevated serum direct bilirubin level, cholestasis has been associated with depigmented stools or cholestatic feces. These findings suggest an extrahepatic obstructive process. Early identification of infants with biliary atresia—a leading cause of cholestasis—is essential for timely intervention and improved outcomes(Feldman & Sokol, 2021). However, a significant clinical limitation is that not all healthcare facilities are equipped to perform a Kasai to achieve optimal surgical outcomes. The purpose of this case report is to describe the improvement of extrahepatic cholestasis after administration of steroids.
Case Study
A 24-day-old infant boy presented to the outpatient clinic for pediatric hepatology with the primary complaint of jaundice since birth, accompanied by acholic stools. Physical examination revealed that he had icteric sclerae, hepatomegaly, with no evidence of splenomegaly. He was born via normal delivery with a birth weight of 2700 grams. He was admitted to the NICU at the age of 3 days due to a lung infection. His laboratory results were aspartate aminotransferase (AST) 397 U/L, Alanine aminotransferase (ALT) 257 U/L, direct bilirubin 23.29 mg/dl, total bilirubin 33.25 mg/ dl, albumin 3.15 g/dl, Gamma-glutamyl transferase (GGT) 65 U/L, and alkaline phosphatase (ALP) 275 U/L. A 2-phase abdominal ultrasound showed pre-prandial gallbladder with the size of 1.57 x 0.8 cm and postprandial gallbladder with the -+size of 0.79 x 0.23cm.
The liver biopsy revealed a dilated portal tract with proliferation of bile ducts, several bile ducts containing bile pigment, accompanied by inflammatory cells, lymphocytes, and neutrophils. Hepatocytes showed ballooning degeneration and cloudy swelling, with some containing dilated intracanalicular bile pigment. Multinucleated giant cell hepatocytes were also observed, and foci of lymphocytes inflammation were present between the hepatocytes. The MT and RC staining showed fibrosis of the portal vein (F1). The conclusion was an extrahepatic obstructive cholestasis, which can be obtained in biliary atresia. The patient was subsequently started on methylprednisolone 2 mg/kg/dose in divided doses, followed by tapering and ursodeoxycholic acid 10 mg/kg/dose 3 times a day. Parents refused the Kasai surgery.
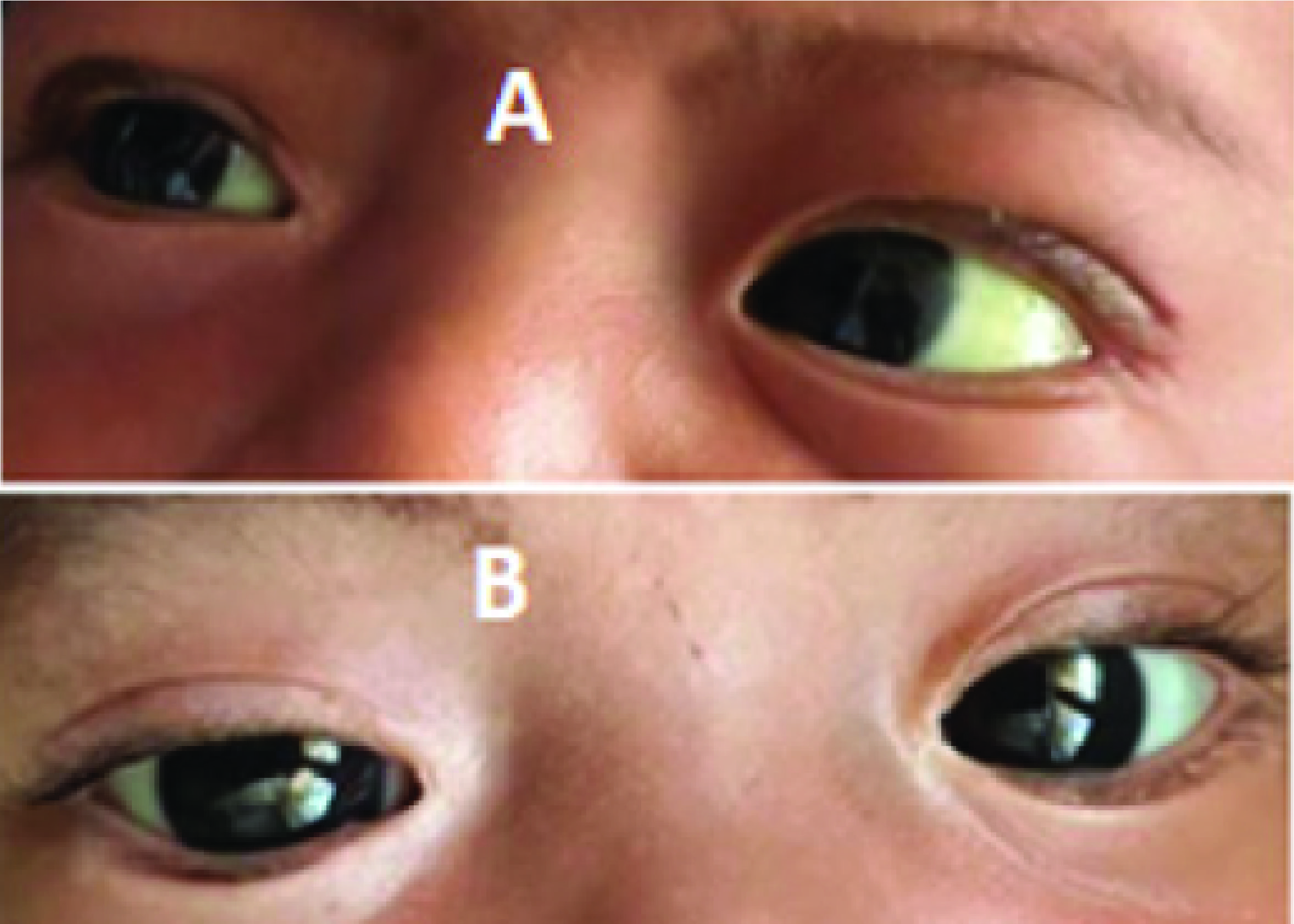
Figure 1.Clinical jaundice of the patient (A. Before therapy and B. After therapy)
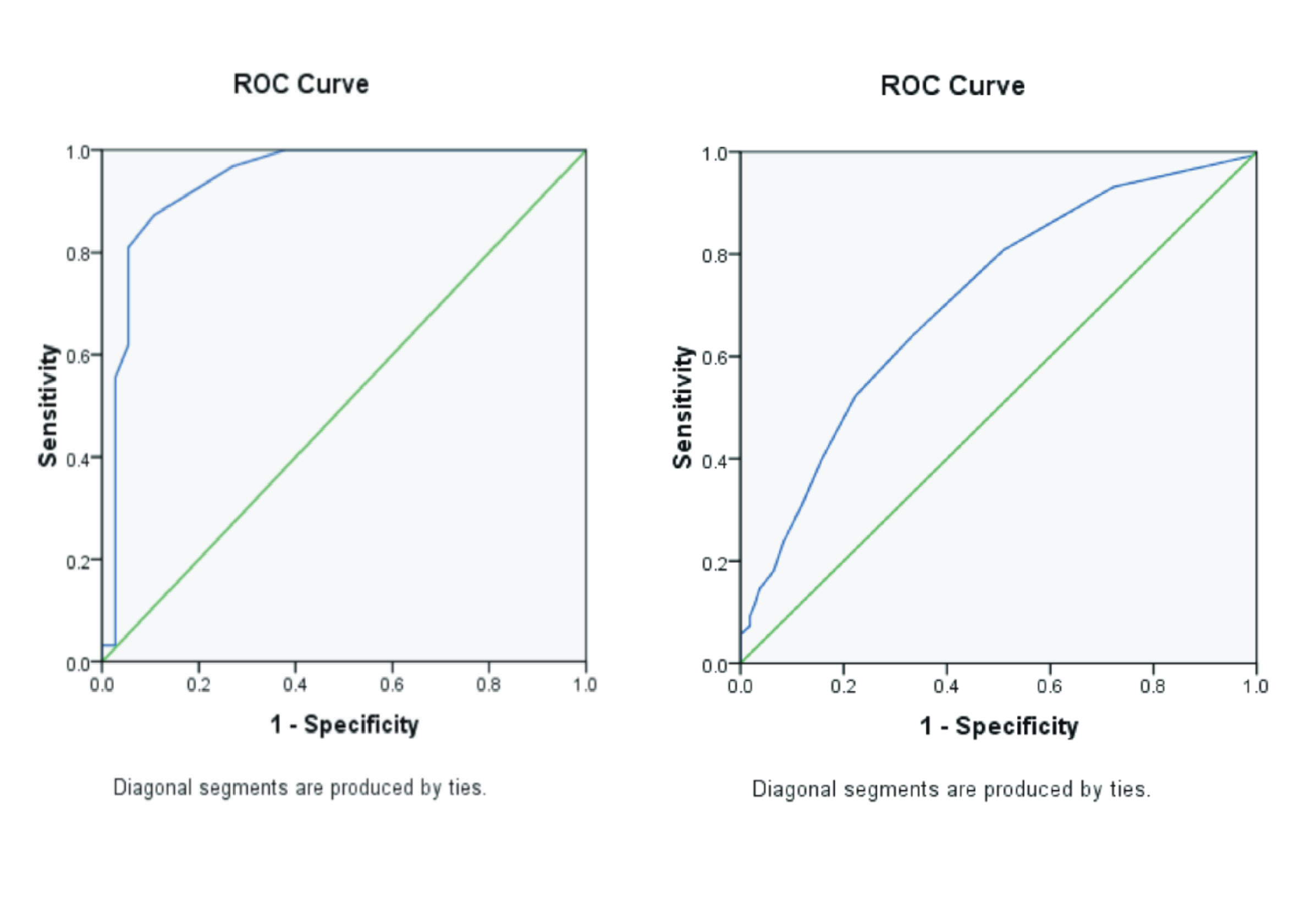
Two months after initiating treatment, the patient showed no jaundice, the stool was yellow and the laboratory results were AST 56 U/L, ALT 82 U/L, Gamma GT 244 U/L, direct bilirubin 0.8 mg/dl, total bilirubin 1.3 mg/dlFigure 1andFigure 2. No recurrence jaundice was observed post-steroid treatment. The stool color was darker than before, followed by an improvement in laboratory valuesFigure 3. The second phase 2 abdominal ultrasound evaluation showed gallbladder measurements of preprandial ±1.4 x 3.19 x 1.4 cm (volume ±3.2 cc), postprandial ±0.95 x 2, 97 x 1.28 cm (volume ±1.87 cc) with a contractility index of 43%Figure 4.
Figure 2.Improvement of laboratory parameters of the patient every two weeks evaluation (A. Bilirubin serum level and B. Liver function test)
Figure 3.Acholic stool improved after therapy. (a) Before therapy and (b) After therapy
Figure 4.Abdominal ultrasonography of the patient. (a) Before therapy and (b) After therapy
Result
Direct and total bilirubin measurements are needed to rule out cholestasis in infants with prolonged jaundice. Cholestasis is established if the serum direct bilirubin level exceeds 1 mg/dl (17.1 μmol/L) when the total serum bilirubin level is less than 5 mg/dl (85.5 μmol/L), or if the serum direct bilirubin level is more than 20% when the total serum bilirubin level is greater than 5 mg/dl (85.5 μmol/L). Cholestasis can be the first sign of severe hepatobiliary impairment(Feldman & Mack, 2012). If cholestasis is confirmed by serum bilirubin measurements, a two-phase ultrasound and biopsy of the liver may be performed. Anintraoperative cholangiogram or ERCP may be performed if the results of this examination are inconclusive(Feldman & Sokol, 2021). Neonatal cholestasis is often caused by biliary atresia, which occurs when the bile ducts become blocked within the first 3 months of life. This condition is quite common, affecting approximately 1 in every 6.000 to 12.000 live births worldwide(Fawaz et al., 2017). Occasionally, other congenital abnormalities, such as malformations at birth, may manifest(Davenport et al., 2006).
Clinically, distinguishing
Aziz, S., Wild, Y., Rosenthal, P., Goldstein, R.B., 2011. Pseudo Gallbladder Sign in Biliary Atresia—An Imaging Pitfall. Pediatric Radiology Vol. 41(5), Pp. 620-626.
Butler, A.E., Schreiber, R.A., Yanchar, N., Emil, S., Laberge, J.-M., Canadian Biliary Atresia Registry, 2016. The Canadian Biliary Atresia Registry: Improving The Care of Canadian Infants with Biliary Atresia. Paediatrics & Child Health Vol. 21(3), Pp. 131-134.
Coutinho, A.E., Chapman, K.E., 2011. The Anti-Inflammatory and Immunosuppressive Effects of Glucocorticoids, Recent Developments and Mechanistic Insights. Molecular and Cellular Endocrinology Vol. 335(1), Pp. 2-13.
Davenport, M., Tizzard, S.A., Underhill, J., Mieli-Vergani, G., Portmann, B., Hadzić, N., 2006. The Biliary Atresia Splenic Malformation Syndrome: A 28-Year Single-Center Retrospective Study. The Journal of Pediatrics Vol. 149(3), Pp. 393-400.
Dong, R., Zhao, R., Zheng, S., 2011. Changes in Epigenetic Regulation of CD4+ T Lymphocytesin Biliary Atresia. Pediatric Research Vol. 70(6), Pp. 555-559.
Fawaz, R., Baumann, U., Ekong, U., Fischler, B., Hadzic, N., Mack, C.L., McLin, V.A., Molleston, J.P., Neimark, E., Ng, V.L., Karpen, S.J., 2017. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition Vol. 64(1), Pp. 154-168.
Feldman, A.G., Mack, C. L., 2012. Biliary Atresia: Cellular Dynamics and Immune Dysregulation, Seminars in Pediatric Surgery Vol. 21(3), Pp. 192-200
Feldman, A.G., Sokol, R.J., 2021. Neonatal Cholestasis: Updates on Diagnostics, Therapeutics, and Prevention. NeoReviews Vol. 22(12), Pp. e819–e836.
Götze, T., Blessing, H., Grillhösl, C., Gerner, P., Hoerning, A., 2015. Neonatal Cholestasis - Differential Diagnoses, Current Diagnostic Procedures, and Treatment. Frontiers in Pediatrics Vol. 3, Pp. 43.
Harpavat, S., Finegold, M.J., Karpen, S.J., 2011. Patients with Biliary Atresia Have Elevated Direct/Conjugated Bilirubin Levels Shortly After Birth. Pediatrics Vol. 128 (6), Pp. e1428-e1433.
Hodgson, J.M., van Someren, V.H., Smith, C., Goyale, A., 2018. Direct Bilirubin Levels Observed in Prolonged Neonatal Jaundice: A Retrospective Cohort Study. BMJ Paediatric Open Vol. 2(1), Pp. e000202.
Lakshminarayanan, B., Davenport, M., 2016. Biliary Atresia: A Comprehensive Review. Journal of Autoimmunity Vol. 73, Pp. 1-9.
Liao, F.-M., Chang, K.-C., Wu, J.-F., Chen, H.-L., Ni, Y.-H., Chang, M.-H., 2022. Direct Bilirubin and Risk of Biliary Atresia. Pediatrics Vol. 149(6), e2021053073.
Lykavieris, P., Chardot, C., Sokhn, M., Gauthier, F., Valayer, J., Bernard, O., 2005. Outcome in Adulthood of Biliary Atresia: A Study of 63 Patients who Survived for Over 20 Years with Their Native Liver. Hepatology Vol. 41(2), Pp. 366-371.
Maisels, M.J., Clune, S., Coleman, K., Gendelman, B., Kendall, A., McManus, S., Smyth, M., 2014. The Natural History of Jaundice in Predominantly Breastfed Infants. Pediatrics Vol. 134(2), Pp. e340-345.
McKiernan, P.J., Baker, A.J., Lloyd, C., Mieli-Vergani, G., Kelly, D.A., 2009. British Paediatric Surveillance Unit Study of Biliary Atresia: Outcome at 13 Years. Journal of Pediatric Gastroenterology and Nutrition Vol. 48(1), Pp. 78-81.
Okajima, K., Nagaya, K., Azuma, H., Suzuki, T., 2016. Biliary Atresia and Stool: Its Consistency and Fat Content, Another Potentially Useful Clinical Information. European Journal of Gastroenterology & Hepatology Vol. 28(1), Pp. 118.
Patel, K.R., 2023. Biliary Atresia and Its Mimics. Diagnostic Histopathology Vol. 29(1), Pp. 52-66.
Russo, P., Magee, J.C., Anders, R.A., Bove, K.E., Chung, C., Cummings, O.W., Finegold, M.J., Finn, L.S., Kim, G.E., Lovell, M.A., Magid, M.S., Melin-Aldana, H., Ranganathan, S., Shehata, B.M., Wang, L.L., White, F.V., Chen, Z., Spino, C., Childhood Liver Disease Research Network (ChiLDReN), 2016. Key Histopathologic Features of Liver Biopsies That Distinguish Biliary Atresia From Other Causes of Infantile Cholestasis and Their Correlation With Outcome: A Multicenter Study. The American Journal of Surgical Pathology Vol. 40(12), Pp. 1601-1615.
Saito, T., Hishiki, T., Terui, K., Mitsunaga, T., Terui, E., Nakata, M., Yoshida, H., 2011. Toll-like Receptor mRNA Expression in Liver Tissue from Patients with Biliary Atresia. Journal of Pediatric Gastroenterology and Nutrition Vol. 53(6), Pp. 620-626.
Setyoboedi, B., Prihaningtyas, R., Utomo, M., Arief, S., 2022. Early Detection of Biliary Atresia in Primary Health Care: Still A Problem. F1000Research Vol. 11, Pp. 1245.
Siddiqui, A.H., Koirala, J., 2024. Methicillin-Resistant Staphylococcus aureus. In: StatPearls. StatPearls Publishing, Treasure Island (FL).
Vejchapipat, P., Poomsawat, S., Chongsrisawat, V., Honsawek, S., Poovorawan, Y., 2012. Elevated Serum IL-18 and Interferon-Gamma in Medium-Term Survivors of Biliary Atresia. European Journal of Pediatric Surgery Vol. 22(1), Pp. 29-33.
Vij, M., Rela, M., 2020. Biliary Atresia: Pathology, Etiology And Pathogenesis. Future Sci OA Vol. 6(5), Pp. FSO466.
Williams, D.M., 2018. Clinical Pharmacology of Corticosteroids. Respir Care Vol. 63(6), Pp. 655-670
Copyright (c) 2025 Journal of Vocational Health Studies

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
- The authors agree to transfer the transfer copyright of the article to the Journal of Vocational Health Studies (JVHS) effective if and when the paper is accepted for publication.
- Legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike (CC BY-NC-SA), implies that publication can be used for non-commercial purposes in its original form.
- Every publications (printed/electronic) are open access for educational purposes, research, and library. Other that the aims mentioned above, editorial board is not responsible for copyright violation.
Journal of Vocational Health Studies is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License