SEQUESTRATION OF ERYTHROCYTE INFECTED BY Plasmodium berghei ANKA IN MICE LIVER TREATED WITH ETHANOL EXTRACT OF PEARL GRASS (Hedyotis corymbosa (L.) Lamk)
Background: Malaria is a parasitic infection caused by the Plasmodium genus. Certain Plasmodium species can evade the immune system by sequestering internal organs, including the liver. The ethanolic extract of pearl grass (Hedyotis corymbosa (L.) Lamk) (EEPG) has been reported to have an antimalarial activity in reducing parasitemia and hepatomegaly in Plasmodium berghei ANKA-infected mice. Purpose: To analyze the effect of EEPG administration on the sequestration of P. berghei ANKA-infected erythrocytes in the livers of BALB/c mice. Method: P. berghei ANKA-infected mice were treated with EEPG at doses of 250, 300, and 350 mg/kg BW. The positive and negative control groups received dihydroartemisinin-piperaquine (DHP) 187.2 mg/kgBW and 1% CMCNa, respectively. The treatments were administered for four consecutive days, followed by observation of parasitemia on Giemsa-stained tail blood smears. On day five, mice were sacrificed for liver removal. Sequestrations were observed on HE-stained slides of mouse livers. The differences in sequestration between treatment groups were analyzed using One-way ANOVA and Games-howell post-hoc analysis. The correlation between parasitemia and sequestration was analyzed using the Pearson correlation test. Result: The percentage reduction of the number of infected erythrocyte sequestration in EEPG-treated groups was 81.74%, 77.72%, and 77.70%, respectively, while the positive control group was 91.14%. Parasitemia was correlated with the number of erythrocytes sequestration (p-value < 0.05). Conclusion: EEPG was able to decrease parasitemia along with the decrease in the number of infected erythrocytes sequestration in the liver. These results indicated that EEPG is
Introduction
Malaria is a disease transmitted through the bite of the female Anopheles mosquito and is caused by protozoa of the genusPlasmodium(W.H.O., 2024). In humans, malaria is commonly caused by five species ofPlasmodium, namely (1)Plasmodium falciparumand (2)Plasmodium vivax(Loy et al., 2017), (3)Plasmodium malariae(Plenderleith et al., 2022), (4)Plasmodium ovale(Okafor & Finnigan, 2025), and (5)Plasmodium knowlesi(Bin Said et al., 2022). Species ofP. falciparumaccount for the highest number of malaria-relateddeaths (99%) compared to other species ofPlasmodium. One of the reasons is the ability of this species to evade the immune system by sequestrating and rosetting. Sequestration refers to a phenomenon where infected erythrocytes accumulate in the brain, liver, and lungs(Belachew, 2018);(Viriyavejakul et al., 2014). Theprocess of sequestration inP. falciparuminfection is mediated by the binding ofP. falciparumerythrocytemembrane protein-1 (PfEMP-1) and its receptors on the surface of endothelial cells of microvasculatures in visceral organs, mainly CD36(Fonager et al., 2012)ICAM-1(Cunningham et al., 2017)VCAM-1, E-selectin, and chondroitin sulfate(Mahamar et al., 2017). Inflammatory responses triggered by ruptured infected erythrocytes further support this process by inducing cytokines such as TNF-α, which increase cytoadhesive endothelium surface protein levels like ICAM-1(Gallego-Delgado et al., 2014);(Storm & Craig, 2014). The phenomenon has been reported to occur in various mouse strains infected with various strains ofPlasmodium berghei(Franke-Fayard et al., 2010);(Wardani et al., 2020). Furthermore, Schizont Membrane-Associated Cytoadherence (SMAC) has been reported as a protein that is involved in the sequestration ofP. berghei-infected erythrocytes in mice(Fonager et al., 2012).
Pearl grass (Hedyotis corymbosa(L.)Lamk) or also known asOldenlandia corymbosais an herbaceous plant of the Rubiaceae family. Phytochemically analysis has shown that it contains phytochemical proteins, polysaccharides, polyphenols, tannins, flavonoids, saponins, steroids, triterpenes, and glycosides(Das et al., 2019). Traditionally, this plant has been used as an antipyretic, anti-inflammatory, antibacterial, diuretic, anti-stomach ulcer, anti-dysentery, postpartum medicine, and for digestive disorders(Soemardji et al., 2015). The antimalarial properties of pearl grass have demonstrated its ability to inhibit the growth ofP. falciparumin an in vitro culture by methanolic extract of pearl grass(Mishra et al., 2009)as well asP. bergheiin BALB/c mice by Ethanolic Extract of Pearl Grass (EEPG) that has been reported previously by our research group(Putri et al., 2023). However, the effect of pearl grass extract on the sequestration of malaria-infected erythrocytes has not been reported either in human or in mouse malaria. This study aimed to analyze the effect of EEPG on the parasitemia and sequestration of erythrocytes infected withP. bergheiANKA in the livers of BALB/c mice.
Material and Method
Preparation of the Ethanolic Extract of Pearl Grass (EEPG)
The EEPG was prepared by maceration of simplicia in 70% ethanol solvent in the Laboratory of Herbal Materia Medika, Batu City, East Java Province, Indonesia. The doses of EEPG used were 250 mg/kgBW, 300 mg/kgBW, and 350 mg/kgBW in 1% CMCNa(Putri et al., 2023).
Research design
This study was an experimental laboratory research employing a post-test only control group design. The experiment was conducted between March 2022 and May 2022 across three different: Departments Biochemistry, Anatomical Pathology and Medical Parasitology, Faculty of Medicine, Universitas Airlangga. Twenty-five mice were injected intraperitoneally with 200 μL ofP. berghei-infected blood obtained from donor mice.
The mice were then randomly divided into five groups. Group 1 - 3 received 250 mg/kgBW, 300 mg/kgBW, and 350 mg/kgBW of EEPG, respectively. Group 4 served as the positive control group and was treated with 187.2 mg/kgBW of dihydroartemisinin-piperaquine (DHP). Group 5 acted as the negative control group and received 1% CMCNa. All treatments were administered orally once a day for four consecutive days.
On day five, a drop of blood was collected from each mouse’s tail to prepare thin blood smears, which were then stained with Giemsa-stain for parasitemia determination. Subsequently, all mice were sacrificed, and their livers were processed into Hematoxylin Eosin (HE)-stained slides for anatomical pathology examination. The number of infected erythrocyte sequestrations was observed within 5 fields of view per microscopy slide(Wardani et al., 2020). The number of infected erythrocytes sequestration reduction due to EEPG administrations was calculated using the following Formula (1). In which NS: number ofP. berghei-infected erythrocytes sequestration, TG: EEPG treated group and NG: negative control group.
\( \documentclass{article} \usepackage{amsmath} \begin{document} \displaystyle \% \text{ Reduction} = \frac{\text{Mean of NS in NG} - \text{Mean of NS in TG}}{\text{Mean of NS in NG}} \times 100\% \quad \text{....(1)} \end{document} \)
Data analysis
The sequestration data were statistically analyzed using a One-way ANOVA followed by Games-howell post-hoc tests. Correlations between variables were assessed using the Pearson correlation test.
Result
Sequestration ofP. bergheiANKA-infected erythrocytes in mice the liver
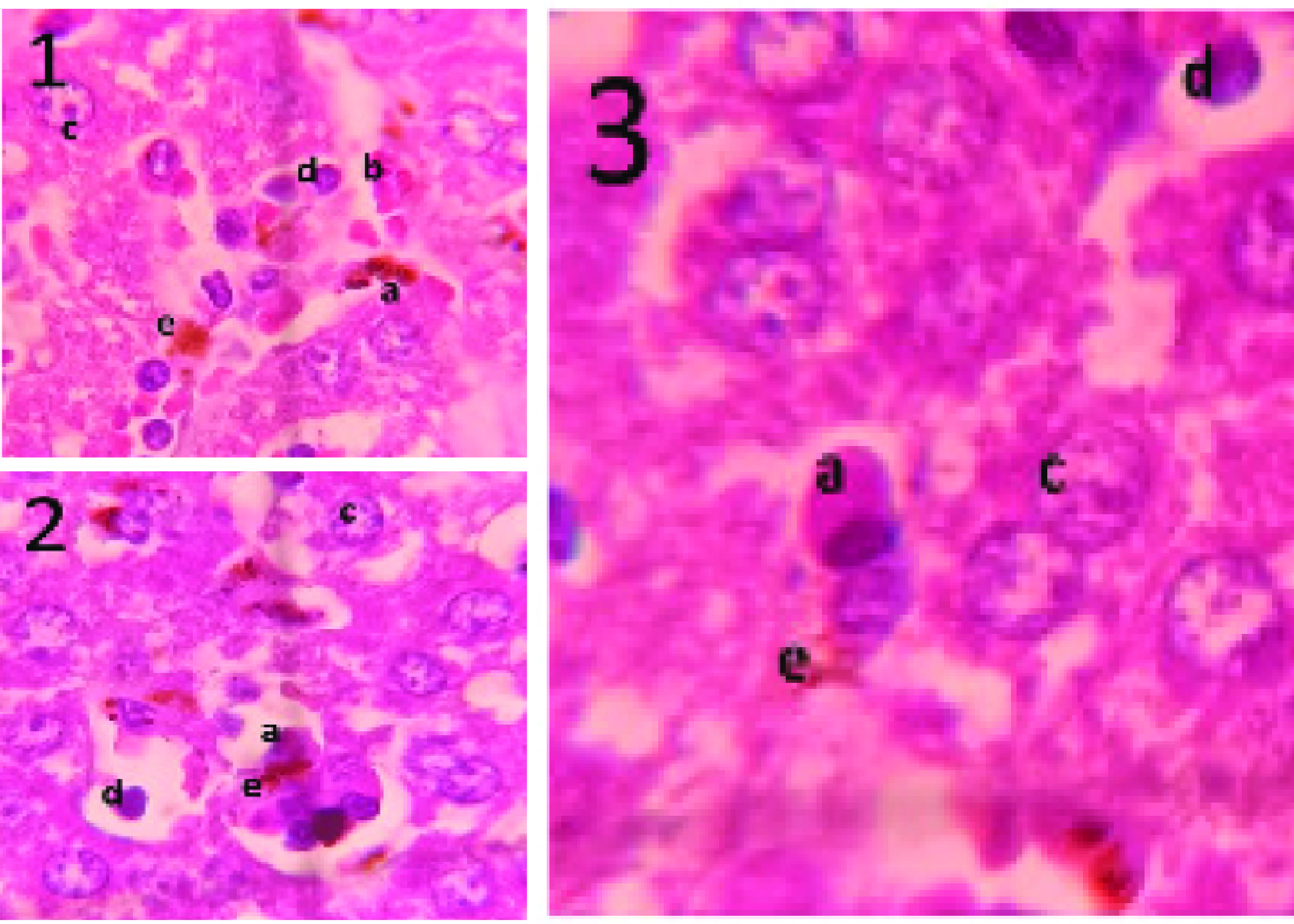
Microscopic observation of the HE-stained mice liver tissues revealed a purple color of the nuclei of the hepatic cells, the clear red of healthy erythrocytes, the purple color of infected erythrocytes containing parasites, and the purple color of various leukocytes. An overview of the HE-stained mice livers is presented inFigure 1. Based onFigure 1, sequestrations ofP. bergheiANKA-infected erythrocytes were observed in the sinusoids of the liver. Infected erythrocytes can be distinguished from healthy erythrocytes
Ali, A.H., Sudi, S., Shi-Jing, N., Hassan, W.R.M., Basir, R., Agustar, H.K., Embi, N., Sidek, H.M., Latip, J., 2021. Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals (Basel, Switzerland) Vol. 14(3), Pp. 248.
Antwi-Baffour, S., Mensah, B.T., Johnson, G., Armah, D.N.O., Ali-Mustapha, S., Annison, L., 2023.Haematological Parameters and Their Correlationwith The Degree of Malaria Parasitaemia amongOutpatients Attending A Polyclinic. Malaria Journal Vol. 22(1), Pp. 281.
Belachew, E.B., 2018. Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites. Journal of Immunology Research Vol. 2018, Pp. 6529681.
Bin Said, I., Kouakou, Y.I., Omorou, R., Bienvenu, A.-L., Ahmed, K., Culleton, R., Picot, S., 2022. Systematic Review of Plasmodium knowlesi in Indonesia: A Risk of Emergence in The Context of Capital Relocation to Borneo ? Parasites & Vectors Vol. 15(1), Pp. 258.
Bruneel, F., 2019. Human Cerebral Malaria: Mini Review. Rev Neurol (Paris) Vol. 175(7-8), Pp. 445-450.
Clark, I.A., Budd, A.C., Alleva, L.M., Cowden, W.B., 2006. Human Malarial Disease: A Consequence of Inflammatory Cytokine Release. Malaria Journal Vol. 5(1), Pp. 85.
Cunningham, D.A., Lin, J., Brugat, T., Jarra, W., Tumwine, I., Kushinga, G., Ramesar, J., Franke-Fayard, B., Langhorne, J., 2017. ICAM-1 is A Key Receptor Mediating Cytoadherence and Pathology in The Plasmodium Chabaudi Malaria Model. Malaria Journal Vol. 16(1), Pp. 185.
Das, S., Mondal, N., Mondal, S., Ghosh, P., Ghosh, C., Das, C., Chatterjee, S., 2019. Botanical Features, Phytochemical and Pharmacological Overviews of Oldenlandia Corymbosa Linn.: A Brief Review. The Pharma Innovation Journal Vol. 8(2), Pp. 464-468.
Fonager, J., Pasini, E.M., Braks, J.A.M., Klop, O., Ramesar, J., Remarque, E.J., Vroegrijk, I.O.C.M., van Duinen, S.G., Thomas, A.W., Khan, S.M., Mann, M., Kocken,C.H.M., Janse, C.J., Franke-Fayard, B.M.D., 2012.Reduced CD36-Dependent Tissue Sequestration of Plasmodium-Infected Erythrocytes is Detrimentalto Malaria Parasite Growth In Vivo. The Journal ofExperimental Medicine Vol. 209(1), Pp. 93-107.
Franke-Fayard, B., Fonager, J., Braks, A., Khan, S.M., Janse, C.J., 2010. Sequestration and Tissue Accumulationof Human Malaria Parasites: Can We Learn Anythingfrom Rodent Models of Malaria? PLoS PathogensVol. 6(9), Pp. e1001032.
Gallego-Delgado, J., Ty, M., Orengo, J., Hoef, D., Rodriguez, A., 2014. A Surprising Role for Uric Acid: The Inflammatory Malaria Response. Current Rheumatology Reports Vol. 16(401), Pp. 1-6.
Gao, F., Lucke-Wold, B.P., Li, X., Logsdon, A.F., Xu, L.-C., Xu, S., LaPenna, K.B., Wang, H., Talukder, M.A.H., Siedlecki, C.A., Huber, J.D., Rosen, C.L., He, P., 2018. Reduction of Endothelial Nitric Oxide Increases The Adhesiveness of Constitutive Endothelial Membrane ICAM-1 through Src-Mediated Phosphorylation. Frontiers in Physiology Vol. 8, Pp. 1-14.
Ghazanfari, N., Mueller, S.N., Heath, W.R., 2018. Cerebral Malaria in Mouse and Man. Frontiers in Immunology Vol. 9, Pp. 1-11.
Hempel, C., Kohnke, H., Maretty, L., Jensen, P.Ø., Staalsø, T., Kurtzhals, J.A.L., 2014. Plasmodium falciparum Avoids Change in Erythrocytic Surface Expression of Phagocytosis Markers during Inhibition of Nitric Oxide Synthase Activity. Molecular and Biochemical Parasitology Vol. 198(1), Pp. 29-36.
Lennartz, F., Smith, C., Craig, A.G., M.K., Higgins., 2019. Structural Insights into Diverse Modes of ICAM-1 Binding by Plasmodium falciparum-Infected Erythrocytes. Proceedings of The National Academy of Science of The United Stated of America Vol. 116(04), Pp. 20124–20134.
Loy, D.E., Liu, W., Li, Y., Learn, G.H., Plenderleith, L.J., Sundararaman, S.A., Sharp, P.M., Hahn, B.H., 2017. Out of Africa: Origins and Evolution of The Human Malaria Parasites Plasmodium falciparum and Plasmodium Vivax. International Journal for Parasitology Vol. 47(2-3), Pp. 87-97.
Mahamar, A., Attaher, O., Swihart, B., Barry, A., Diarra, B.S., Kanoute, M.B., Cisse, K.B., Dembele, A.B., Keita, S., Gamain, B., Gaoussou, S., Issiaka, D., Dicko, A., Duffy, P.E., Fried, M., 2017. Host Factors That Modify Plasmodium falciparum Adhesion to Endothelial Receptors. Scientific Reports Vol. 7(1), Pp. 13872.
Mishra, K., Dash, A.P., Swain, B.K., Dey, N., 2009. Anti-Malarial Activities of Andrographis paniculata and Hedyotis corymbosa Extracts and Their Combination with Curcumin. Malaria Journal Vol. 8, Pp. 26.
Oelschlegel, A.M., Bhattacharjee, R., Wenk, P., Harit, K., Rothkötter, H.-J., Koch, S.P., Boehm-Sturm, P., Matuschewski, K., Budinger, E., Schlüter, D., Goldschmidt, J., Nishanth, G., 2024. Beyond The Microcirculation: Sequestration of Infected Red Blood Cells and Reduced Flow in Large Draining Veins in Experimental Cerebral Malaria. Nature Communications Vol. 15(1), Pp. 2396.
Okafor, C.N., Finnigan, N.A., 2025. Plasmodium Ovale Malaria. In: StatPearls. StatPearls Publishing, Treasure Island (FL).
Plenderleith, L.J., Liu, W., Li, Y., Loy, D.E., Mollison, E., Connell, J., Ayouba, A., Esteban, A., Peeters, M., Sanz, C.M., Morgan, D.B., Wolfe, N.D., Ulrich, M., Sachse, A., Calvignac-Spencer, S., Leendertz, F.H., Shaw, G.M., Hahn, B.H., Sharp, P.M., 2022. Zoonotic Origin of The Human Malaria Parasite Plasmodium malariae from African Apes. Nature Communications Vol. 13(1), Pp. 1868.
Putri, J., Chairunnisa, N., Arwati, H., Kahar, H., 2023. Effect of Ethanol Extract of Hedyotis corymbosa (L.) Lamk against Parasitemia and Hepatomegaly in Plasmodium berghei ANKA-Infected Mice. Qanun Medika - Medical Journal Faculty of Medicine Muhammadiyah Surabaya Vol. 7(1), Pp. 89-99.
Rai, S., Girdhar, M., Siraj, F., Sharma, S., Kumar, M., Katyal, A., 2023. Mechanistic Insights into Immunopathogenesis of Murine Cerebral Malaria: Cues from “Young” C57BL/6J and BALB/c Mice. Immunology Letters. Vol. 256–257, Pp. 9-19.
Sahat, D., 2006. Pengaruh Pemberian Ekstrak Hedyotis corymbosa Dosis Bertingkat terhadap Produksi Nitric Oxide Makrofag Mencit BALB/C yang diinfeksi dengan Salmonella typhimurium (Thesis). Universitas Diponegoro, Faculty of Medicine, Department of Medicine.
Schober, P., Boer, C., Schwarte, L.A., 2018. Correlation Coefficients: Appropriate Use and Interpretation. Anesthesia and Analgesia Vol. 126(5), Pp. 1763-1768.
Serirom, S., Raharjo, W.H., Chotivanich, K., Loareesuwan, S., Kubes, P., Ho, M., 2003. Anti-Adhesive Effect of Nitric Oxide on Plasmodium falciparum Cytoadherence under Flow. The American Journal of Pathology Vol. 162(5), Pp. 1651-1660.
Soemardji, A., Anisa, I., Damayanti, N., 2015. Study on Rumput Mutiara (Hedyotis corymbosa) Herbs as Medicine. Journal Of Medicine & Health Vol. 1(2), Pp. 187-199.
Storm, J., Craig, A.G., 2014. Pathogenesis of Cerebral Malaria-Inflammation and Cytoadherence. Frontiers in Cellular and Infection Microbiology Vol. 4, Pp. 100.
Vasquez, M., Zuniga, M., Rodriguez, A., 2021. Oxidative Stress and Pathogenesis in Malaria. Frontiers in Cellular and Infection Microbiology Vol. 11, Pp. 768182.
Viriyavejakul, P., Khachonsaksumet, V., Punsawad, C., 2014. Liver Changes in Severe Plasmodium falciparum Malaria: Histopathology, Apoptosis and Nuclear Factor Kappa B Expression. Malaria Journal Vol. 13(1), Pp. 106.
Wardani, K., Aini, K., Arwati, H., Sandhika, W., 2020. Sequestration of Erythrocytes Infected with Plasmodium berghei ANKA in BALB/c Mice Treated with Goat Bile. Qanun Medika - Medical Journal Faculty of Medicine Muhammadiyah Surabaya Vol. 4(2), Pp. 179.
Wassmer, S.C., Grau, G.E.R., 2017. Severe Malaria: What’s New on The Pathogenesis Front? International Journal for Parasitology Vol. 47(2-3), Pp. 145-152.
Weinberg, J.B., Lopansri, B.K., Mwaikambo, E., Granger, D.L., 2008. Arginine, Nitric Oxide, Carbon Monoxide, and Endothelial Function in Severe Malaria. Current Opinion in Infectious diseases Vol. 21(5), Pp. 468-475.
WHO, 2024. Fact Sheet about Malaria.Zuniga, M., Gomes, C., Chen, Z., Martinez, C., Devlin, J.C., Loke, P., Rodriguez, A., 2022.
Zuniga, M., Gomes, C., Chen, Z., Martinez, C., Devlin, J.C., Loke, P., Rodriguez, A., 2022. Plasmodium Falciparum and TNF-α Differentially Regulate Inflammatory and Barrier Integrity Pathways in Human Brain Endothelial Cells. mBio Vol. 13(5), Pp. e0174622.
Copyright (c) 2025 Journal of Vocational Health Studies

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
- The authors agree to transfer the transfer copyright of the article to the Journal of Vocational Health Studies (JVHS) effective if and when the paper is accepted for publication.
- Legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike (CC BY-NC-SA), implies that publication can be used for non-commercial purposes in its original form.
- Every publications (printed/electronic) are open access for educational purposes, research, and library. Other that the aims mentioned above, editorial board is not responsible for copyright violation.
Journal of Vocational Health Studies is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License