OPTIMIZATION OF DENATURATION TEMPERATURE AND TIME USING REAL-TIME PCR METHOD IN HEPATITIS B TEST
Downloads
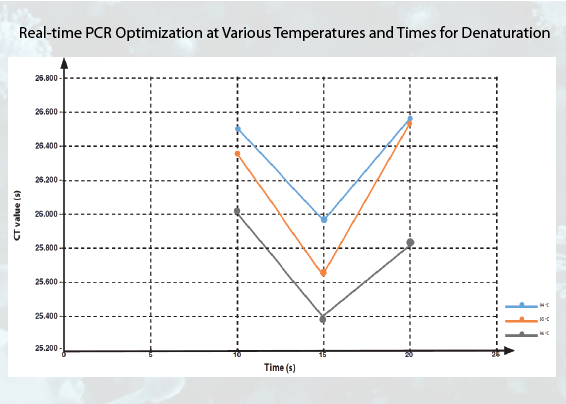
Background: The hepatitis B test can be conducted using the real-time PCR method. In chronic cases, the test is performed by detecting one of the Hepatitis B Virus (HBV) specific genes, HBcAg. The real-time PCR method requires optimization to obtain optimal results. Purpose: This research aims to determine the optimal temperature and time for denaturation in the hepatitis B test using the real-time PCR method. Method: The research method used was a quasi-experiment involving variations in temperature (94 °C, 95 °C, and 96 °C) and time (10 seconds, 15 seconds, and 20 seconds) for denaturation. Data processing resulted in static group comparisons based on 27 primary data from the Cycle Threshold (CT) value. Result: Variations in temperature conditions, specifically at 94 °C, 95 °C, and 96 °C, combined with a denaturation time of 10 seconds, yielded the mean CT values of 26.495, 26.355, and 26.003, respectively. When the temperature conditions were maintained at 94 °C, 95 °C, and 96 °C, with a denaturation time of 15 seconds, yielded the mean CT values of 25.962, 25.641, and 25.396. Similarly, under temperature conditions of 94 °C, 95 °C, and 96 °C with a denaturation time of 20 seconds, yielded the mean CT values of 26.544, 26.505, and 25.830 were obtained. Conclusion: Optimal results in this study are obtained through the acquisition of the smallest CT value, namely at temperature conditions of 96 °C and 15 seconds.
Amanda, K., 2020. Optimasi Suhu Annealing Proses PCR Amplifikasi Gen shv Bakteri Eschericia coli Pasien Ulkus Diabetik. Jurnal Mahasiswa Farmasi Fakultas Kedokteran UNTAN Vol. 4(1), Pp. 1-6.
Bhakti, T.S., 2022. Optimasi Kit Zeesan SARS-COV-2 Test Kit untuk Pengujian Deteksi DNA Porcine pada Produk Olahan Daging dan Produk Olahan Kompleks. BiosciED: Journal of Biological Science and Education Vol. 3(1), Pp. 1-8.
DNA-Technology, 2021. HBV Quantitative REAL-TIME PCR Kit (PREP-NA DNA/RNA Extraction Kit included).
Eppendorf, 2020. Community - Eppendorf Handling Solutions. URL https://handling-solutions.eppendorf.com/TEXT (accessed 1.29.24).
Fatchiyah, Arumingtyas, E.L., Widyarti, S., Rahayu, S., 2018. Biologi Molekular : Prinsip Dasar Analisis, 1st ed. Erlangga, Jakarta.
Ghosh, M., Nandi, S., Dutta, S., Saha, M.K., 2015. Detection of Hepatitis B Virus Infection: A Aystematic Review. World J Hepatol Vol. 7(23), Pp. 2482-2491.
ICTV, 2021. Genus: Orthohepadnavirus | ICTV. In: Hepadnaviridae. ICTV. Virus Taxonomy.
Irianto, K., 2014. Bakteriologi, Mikologi & Virologi Panduan Medis & Klinis, 1st ed. Alfabeta, Bandung.
Kimura, T., Rokuhara, A., Sakamoto, Y., Yagi, S., Tanaka, E., Kiyosawa, K., Maki, N., 2002. Sensitive Enzyme Immunoassay for Hepatitis B Virus Core-Related Antigens and Their Correlation to Virus Load. J Clin Microbiol Vol. 40(2), Pp. 439-445.
Korner, K., 2017. PCR Optimization 2.0—The Denaturation Temperature. GEN-Genetic Engineering and Biotechnology News. URL https://www.genengnews.com/insights/pcr-optimization-2-0/ (accessed 1.29.24).
Long, S., Pickering, L., Charles Prober, 2012. Principles and Practice of Pediatric Infectious Diseases. Churchill Livingstone.
Lorenz, T.C., 2012. Polymerase Chain Reaction: Basic Protocol Plus Troubleshooting and Optimization Strategies. J Vis Exp Pp. e3998.
Luckenbaugh, L., Kitrinos, K.M., Delaney, W.E., Hu, J., 2015. Genome-free Hepatitis B Virion Levels in Patient Sera as A Potential Marker to Monitor Response to Antiviral Therapy. J Viral Hepat Vol. 22(6), Pp. 561-570.
Malau, P.M.R., Wicaksono, I.A., 2021. Aktivitas Anti Virus Hepatitis B pada Beberapa Tanaman dengan Metode Pengujian In Vivo dan In Vitro: Review Jurnal. Farmaka Vol. 18(2), Pp. 173-190.
Merdekawati, F., Nurhayati, B., 2023. Desain Primer Gen Pengkode RNA Dependent RNA Polimerase (RdRp) untuk Deteksi Sars Cov2 dengan menggunakan Real Time Polymerase Chain Reaction. Jurnal Riset Kesehatan Poltekkes Depkes Bandung Vol. 15(1), Pp. 30–36.
Ministry of Health, 2021. Profil Kesehatan Indonesia.
Ministry of Health Regulation, 2015. Penanggulangan Hepatitis Virus. 53
Ministry of Health Regulation, 2017. Eliminasi Penularan Human Immunodeficiency Virus, Sifilis, dan Hepatitis B dari Ibu ke Anak. 52.
Monjezi, R., Tan, S.W., Tey, B.T., Sieo, C.C., Tan, W.S., 2013. Detection of Hepatitis B Virus Core Antigen by Phage Display Mediated TaqMan Real-Time Immuno-PCR. J Virol Methods Vol. 187(1), Pp. 121-126.
Raihan, R., Tabassum, S., Al-Mahtab, M., Nessa, A., Jahan, M., Shamim Kabir, C.M., Kamal, M., Cesar Aguilar, J., 2015. Hepatitis B Core Antigen in Hepatocytes of Chronic Hepatitis B: Comparison between Indirect Immunofluorescence and Immunoperoxidase Method. Euroasian J Hepatogastroenterol Vol. 5(1), Pp. 7-10.
Rosida, A., 2016. Pemeriksaan Laboratorium Penyakit Hati. Berkala Kedokteran Vol. 12(1), Pp. 123-131.
Rybicki, E., 2014. A Manual of Online Molecular Biology Techniques.
Sadewa, A.H., 2022. Dasar-Dasar Real-Time PCR - LPPT UGM.
Sastrawinata, Ucke Sugeng, 2008. Virologi Manusia, 2 nd. ed. Alumni Publisher, Bandung.
Siswanto, Y.P., Merdekawati, F., Ernawati, E., Hardiana, A.T., Kurniawan, E., 2019. Optimasi Suhu Anneal-ing dan Konsentrasi Primer untuk Deteksi Brugiamalayi menggunakan Real-Time PCR. Jurnal Riset Kesehatan Poltekkes Depkes Bandung Vol. (1), Pp. 314-321.
Soedarto, 2019. Parasitologi Klinik, 1 st. ed. Airlangga University Press, Surabaya.
Solange, N.K., Alphonsine, M.-K., Moussa, D., Coulibaly N’Golo, D., Syndou, M., Aboubacar, S., Serge, A., Faye-Kette, H., Mireille, D., 2014. Real Time PCR for Viral Load Quantification of Hepatitis Virus B in Côte d’Ivoire, West Africa. Journal of Microbiology Research and Reviews Vol. 2(4), Pp. 34-39.
Thapa, B.R., Walia, A., 2007. Liver function Tests and Their Interpretation. Indian J Pediatr Vol. 74(7), Pp. 663-671.
Thermofisher, 2023. PCR Methods.
Wijayanti, I., 2016. Efektivitas HBsAg – Rapid Screening Test. Jurnal KesMaDaSka Pp. 29-34.
Yusuf, Z., 2010. Polymerase Chain Reaction (PCR). Jurnal Sainstek Vol. 5(2).
Copyright (c) 2024 Journal of Vocational Health Studies

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
- The authors agree to transfer the transfer copyright of the article to the Journal of Vocational Health Studies (JVHS) effective if and when the paper is accepted for publication.
- Legal formal aspect of journal publication accessibility refers to Creative Commons Attribution-NonCommercial-ShareAlike (CC BY-NC-SA), implies that publication can be used for non-commercial purposes in its original form.
- Every publications (printed/electronic) are open access for educational purposes, research, and library. Other that the aims mentioned above, editorial board is not responsible for copyright violation.
Journal of Vocational Health Studies is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License