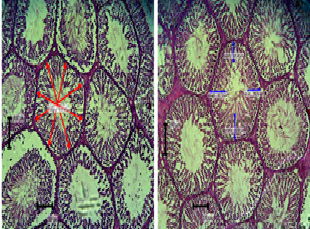
Spermiogramic parameters of Japanese quails (Coturnix coturnix japonica) to aqueous administration of egg lime molasses mixture
Downloads
As demand for animal protein rises, raising Japanese quail holds much potentials in bridging the gap. Phytogenics has been reported to have effect on spermiogramic parameters of animals. This research was done to ascertain the spermiogramic parameters of Japanese quails to administration of aqueous solution of egg lime molasses mixture (ELM). Fresh eggs were placed in a dish, followed by 500g of molasses and 1 liter of lime juice. It was then covered for 10 days and the entire mixture was blended. Two hundred day old Japanese quails were assigned to five treatments in a Completely Randomized Design and were subdivided into 4 replicates of 10 birds each. The control (T1) having no administration of ELM, T2 had an inclusion level of 10 mL, T3: 20 mL, T4: 30 mL and T5: 40 mL, all in 500 mL of water. Feed and water were provided ad libitum. The study was carried out for 49 days. Data were collected on genitalia morphometry and fertilizing potentials. The birds administered 20 mL of ELM had significantly (p <0.05) higher left epididymis weight (0.36 ± 0.05g) compared to the other groups. The inclusion of ELM in the water significantly influenced (p <0.05) left epididymis volume, paired epididymis volume, left epididymis density, paired epididymis density and spermatozoa reserves in the right epididymis. Testosterone values significantly increased (p <0.05) with increased ELM inclusion. It can be concluded that the administration of ELM did not alter growth parameters however birds that received 20 mL per 500 mL of water had the best reproductive parameters.
Adeyemo GO, Longe, Adejumo DO. 2007. The reproductive performance of breeder cocks fed cotton seed cake-based diets. Int J Poult Sci. 6: 140-4.
Ahemen T, Abu AH, Akuba JO. 2016. Effect of Gmelina arborea leaf meal on sperm production and sperm reserves in rabbit bucks. Int J Livest Res. 6: 98-104.
Aire, T.A. 1982. The rete testis of birds. J Anat. 135: 97-100.
Akintunde AO, Ndubuisi-Ogbonna LC, Olorunfemi OA, Ladele MM, Shobo BA, Adewole SA, Akinboye OE. 2023. Nutritional and ethno-medicinal potentials of egg-lime-molasses mixture in livestock production. Asian J Dairy Food Res. 2023: 1-9.
Akintunde AO, Ndubuisi-Ogbonna LC, Olorunfemi OA., Ladele MM, Shobo BA, Adewole SA, Akinboye OE. 2022. Nutritional and ethno-medicinal potentials of egg-lime-molasses mixture in livestock production. Asian J Dairy Food Res. doi: 10.18805/ajdfr.DRF-285
Akintunde AO, Toye AA, Ademola AA, Chimezie VO, Ajayi OA. 2020. Sperm characteristics of Nigeria local cocks and exotic strain of cocks fed graded levels of Moringa oleifera seed meal. Trop Anim Prod Invest. 23: 1-10.
Akintunde AO, Toye AA, Ademola AA, Chimezie VO, Ajayi OA. 2021. Genotype-diet effect on comparative semen parameters of chickens fed graded levels of Moringa oleifera seed meal. Malaysi J Anim. Sci. 24: 41-55.
Akintunde AO, Toye AA. 2021. Egg characteristics and prediction of egg weight in chickens fed graded levels of Moringa oleifera seed meal. Nigerian J Genet. 35: 141-51.
Akintunde AO. 2018. Response of chicken genotypes to dietary levels of Moringa oleifera (Lamarck) seed meal. Ph.D. thesis. Department of Animal Production, University of Ilorin. Ilorin, Nigeria.
Akinyemi MO, Aina AJ, Ewuola EO, Osaiyuwu OH, Ajao EO. 2014. Relationships between body morphometrics and testicular biometrics of West Africa dwarf bucks in Southwestern Nigeria. Int J Agric Biosc. 3: 283-7.
Al-Qarawi AA, Abdel-Rahman HA, El-Belely MS, El-Moug SA. 2001. Intratesticular morphometric, cellular and endocrine changes around the pubertal period in dromedary camels. Vet J. 162: 241-9.
Artoni SMB. 1993. Consideraçíµes sobre a morfologia e a histofisiologia do testículo da codorna (Coturnix cournix japonica). Tese de Doutorado em Anatomia. Instituto de Biociíªncias, Universidade Estadual Paulista. Botucatu-Sí£o Paulo, Brazil.
Aviagen. 2018. Ross broiler performance objectives
Banihani SA. 2019. Testosterone in males as enhanced by onion (Allium cepa L.). Biomolecules 9: 75.
Bielli A, Pedrana G, Gastel MT, Castrillejo A, Moraña A, Lundeheim N, Forsberg M, Rodriguez-Martinez H. 1997. Influence of nutrition on seasonal variations in testicular morphology and function in Corriedale rams. J Reprod Dev. 43: 171-80.
Bull LM, Fernandas MR, Martins B, Cesario DM, Pdovani R, Mendes AA. 2007. Anatomical study on domestic fowl (Gallus domesticus) reproductive system. Int J Morphol. 25: 709-16.
Chimezie VO, Akintunde AO, Ademola AA, Aina FA. 2022. Principal component analysis of bodyweight and morphometric traits in Japanese Quail (Coturnix coturnix japonica). Aceh J Anim Sci. 7: 47-52.
Clulow J, Jones RC. 1982. Production, transport, maturation, storage and survival of spermatozoa in the male Japanese quail, Coturnix coturnix. J Reprod Fertil. 64: 259-66.
Department of Agriculture, Fisheries and Forestry (DAFF). 2013. Structure and dynamic of quails. Commonwealth of Australia. 1-5.
Daramola JO, Adeloye AA, Akintunde AO, Imam TK, Iyasere OS, Sobayo RA. 2010. Effect of Uromaiz on sperm characteristics in West African Dwarf bucks. J Agric Sci Environ. 10: 59-67.
Egbunike GN, Holtz W, Smidt D. 1976. Reproductive capacity of German Landrace boars. II: Sperm production rates as determined by quantitative testicular histology and fron gonadal sperm reserves. Zuchthygiene 11: 35-7.
Ewuola EO, Oyodele DM, Akinyemi DE. 2015. Genitalia morphometry and testicular characteristics of male white Japanese quails at three different age groups. Bull Anim Health Prod Afr. 63: 179-86.
Ezekwe AG. 1998. Gonadal and extragonadal sperm reserve and testicular histometry of post pubertal Muturu bulls. Niger J Anim Prod. 25: 106-10.
França LR, Godinho CL. 2003. Testis morphometry, seminiferous epithelium cycle length and daily sperm production in domestic cats (Felis catus). Biol. Reprod. 68: 1554-61.
Gage MJ, Freckleton RP. 2003. Relative testis size and sperm morphometry across mammals: No evidence for an association between sperm competition and sperm length. Proc R Soc B: Biol Sci. 270: 625-32.
Gbore FA, Egbunike GN. 2008. Testicular and epididymal sperm reserves and sperm production of pubertal boars fed dietary fumonisin B1. Anim Reprod Sci. 105: 392-7.
Hess RA, Thurston RJ, Biellier HV. 1976. Morphology of the epididymal region and ductus deferens of the turkey (Meleagris gallopavo). J Anat. 122: 241-52.
Kumaran LDS, Turner CW. 1949. The normal development of testis in the white Plymouth rock. Poult Sci. 28: 511-20.
Marvan F. 1969. Postnatal development of the male genital tract of the Gallus domesticus. Anat Anz Bd. 124: 443-62.
Moniem KA, Tingari MD, Künzel E. 1980. The fine structure of the boundary tissue of seminiferous tubule of the camel (Camelus dromedarius). Acta Anat. 107: 169-76.
National Veterinary Research Institute (NVRI). 1994. Farmer training on quail production & health management. National Veterinary research Institute. Vom, Nigeria. 44.
Ndelekwute EK, Uzegbu HO, Igwe IR, Nosike RJ, Odoemelam VU, Inyan UO. 2010. Effects of administration of molasses through drinking water on growth and conformation parameters of meat-type chicken. Anim Prod Res Adv. 6: 30-4.
Ogbuewu IP, Okoli IC, Iloeje MU. 2009. Semen quality characteristics, reaction time, testis weight and seminiferous tubule diameter of buck rabbits fed neem (Azadirachta indica, A. juss) leaf meal based diets. Iran J Reprod Med. 7: 23-8.
Osinowo OA, Buvanendram V, Koning NL. 1981. Growth and testicular development in Bunaji bulls. J Anim Prod Res. 1: 55-67.
Oyeyemi MO, Okediran BS. 2007. Testicular parameters and sperm morphology of chinchilla rabbits fed with different planes of soy meal. Int J Morphol. 25: 139-44.
Perry G, Petterson D. 2001. Determining reproductive fertility in herd bulls. University of Missouri Agriculture publication 2011 :1-8.
Reviers M. 1971. Le développement testiculaire chez le coq. I – Croissance ponderale des testicules et développement des tubes séminifères. Ann Biol Anim Bioch Biophys. 11: 519-30.
Steele RGD, Torrie JH. 1990. Principles and procedures of statistics. 2nd Ed. McGraw-Hill Book Co Inc., New York, USA.
Steinberger E, Root A, Ficher M, Smith KD. 1973. The role of androgens in the initiation of spermatogenesis in man. Clin Endocrinol Met. 37: 746-51.
Copyright (c) 2023 Adeyinka Oye Akintunde, Lois Chidinma Ndubuisi-Ogbonna, Mofiyinfoluwa M. Ladele, Oladapo Ayodeji Olorunfemi, Olayinka Abosede Ojo, Samson O. Oyewumi, Bolatito Adenike Shobo, Olufunso Emmanuel Akinboye

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Ovozoa by Unair is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
1. The journal allows the author to hold the copyright of the article without restrictions.
2. The journal allows the author(s) to retain publishing rights without restrictions
3. The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution Share-Alike (CC BY-SA).
4. The Creative Commons Attribution Share-Alike (CC BY-SA) license allows re-distribution and re-use of a licensed work on the conditions that the creator is appropriately credited and that any derivative work is made available under "the same, similar or a compatible license”. Other than the conditions mentioned above, the editorial board is not responsible for copyright violation.