Date Log
Copyright (c) 2025 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
The Characteristics of Chitosan Derived from Lobster Shells and its Effect on Fungi Activity and Water Stability of Lobster Pellets
Corresponding Author(s) : Muhsinul Ihsan
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 17 No. 2 (2025): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
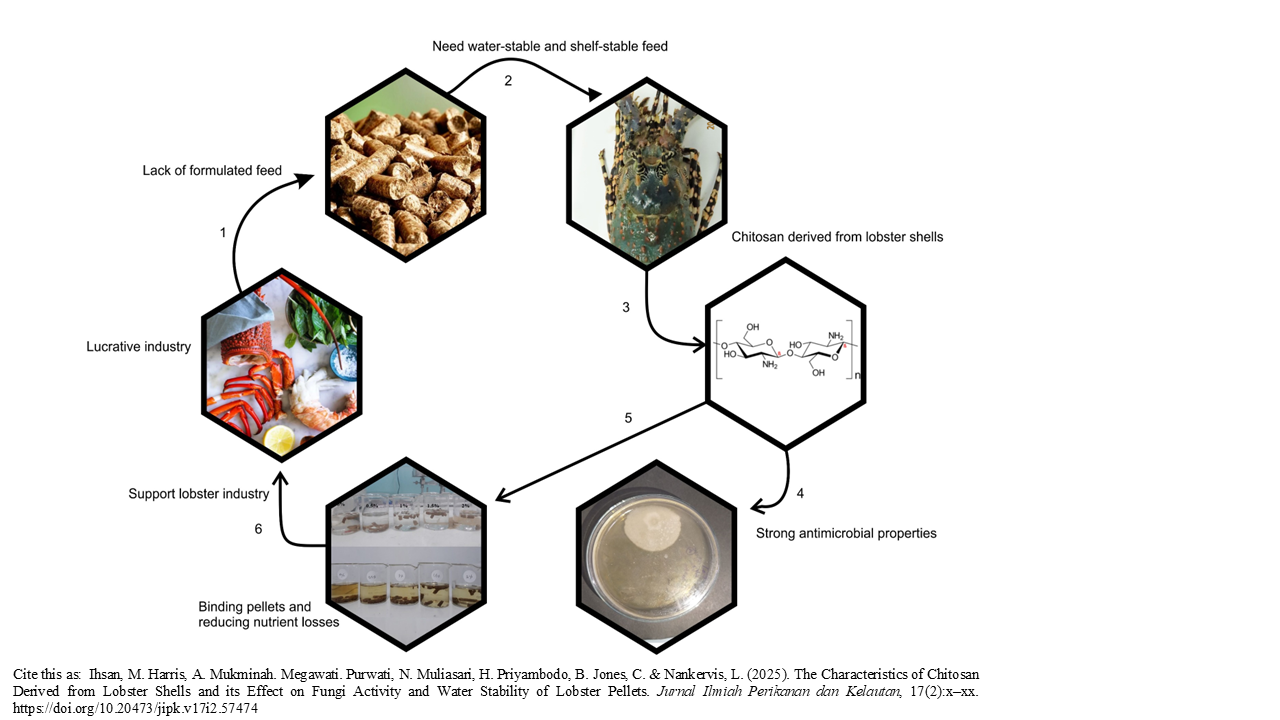
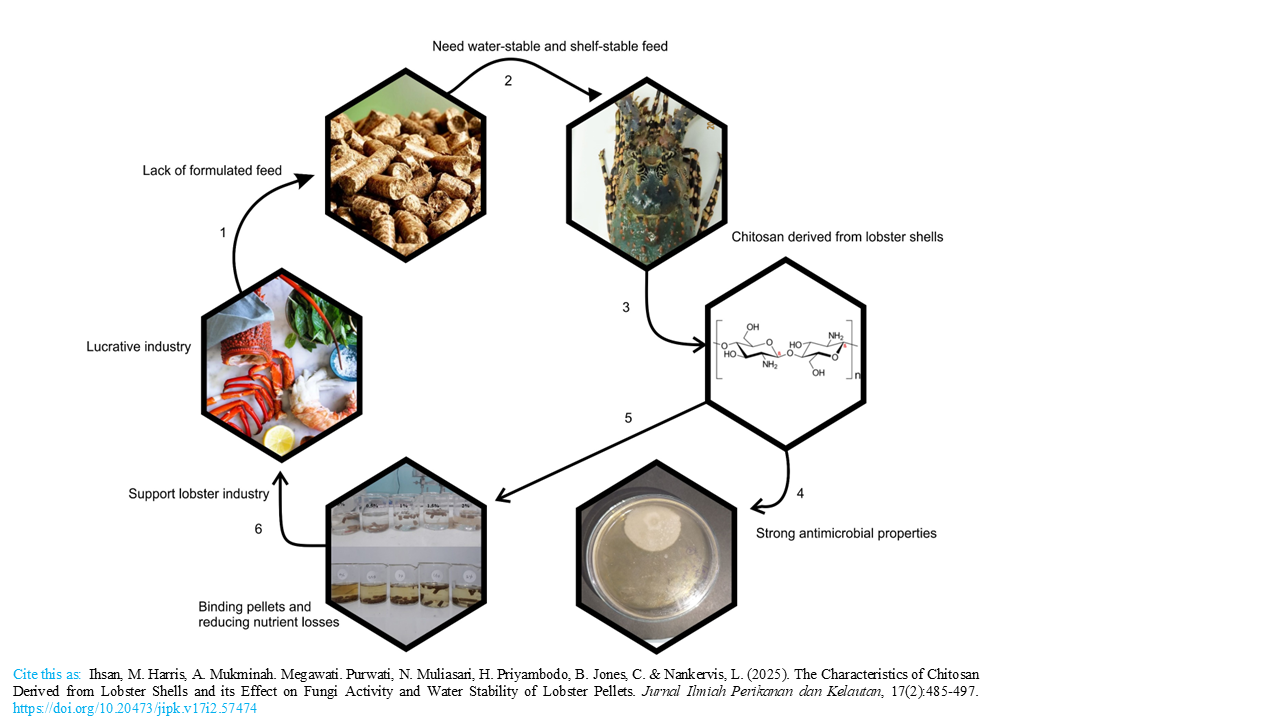
Graphical Abstract
Highlights of Research
- The chitosan was successfully produced from lobster shells Panulirus homarus.
- The characteristics and quality of chitosan from lobster shells Panulirus homarus were observed.
- The anti-fungal activity of chitosan was assessed.
- Chitosan enhances the water stability of lobster pellets.
Abstract
Tropical rock lobster aquaculture is a lucrative industry that is currently limited by the lack of appropriate formulated feed. Its nocturnal, benthic feeding behavior necessitates a water-stable feed that maintains integrity under tropical marine conditions without degrading. Chitosan, a biopolymer derived from lobster (Panulirus homarus) shells, has potential applications in aquaculture as an antifungal agent and feed binder. We report on the characteristics of chitosan extracted from the exoskeleton of spiny lobsters (Panulirus homarus), including its effect on fungal activity and water stability of pellets. Chitosan was produced through three main steps: deproteination, demineralization, and deacetylation. The resulting chitosan was characterized through crude composition (AOAC methods), FTIR spectra, and scanning electron microscope (SEM), while antifungal activity was assessed through in vitro assays. Chitosan was used to coat lobster feed pellets by immersion method at different concentrations (0%, 0,5%, 1%, 1,5%, and 2%), and its impact on pellet water stability was assessed. There were three replications in fungal activity and water stability test. The yield of chitosan was 5.9 ± 0.01% of the total shell mass, with 96.99% ± 0.01 degree of deacetylation (DD). The resulting product contained 5.94 ± 0.07% moisture, 36.72 ± 0.05% ash and 2.73 ± 0.08% nitrogen. Chitosan morphology was characterized as an irregular shape with dimensions ranging from 157 to 391 µm, with a combination of striated surface textures. Increasing concentration of chitosan increased water stability of pellets up to 1.5% inclusion, while 0.5% optimized Fusarium sp. inhibition. These findings suggest that chitosan from lobster shells can be sustainably utilized to enhance feed quality, reducing fungal contamination and nutrient leaching in aquaculture systems.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Abdel-Razek, N. (2019). Antimicrobial activities of chitosan nanoparticles against pathogenic microorganisms in Nile tilapia, Oreochromis niloticus. Aquaculture International, 27(5):1315-1330.
- Adamczuk, A., Kercheva, M., Hristova, M., & Jozefaciuk, G. (2021). Impact of chitosan on water stability and wettability of soils. Materials, 14(24):1-12.
- Ahyat, N. M., Mohamad, F., Ahmad, A., & Azmi, A. A. (2017). Chitin and chitosan extraction from Portunis pelagicus. Malaysian Journal of Analytical Sciences, 21(4):770-777.
- Aly, S. M., Eissa, A. E., Abdel-Razek, N., & El-Ramlawy, A. O. (2023). The antibacterial activity and immunomodulatory effect of naturally synthesized chitosan and silver nanoparticles against Pseudomonas fluorescence infection in Nile tilapia (Oreochromis niloticus): an in vivo study. Fish & Shellfish Immunology, 135(1):108628-108628.
- Amine, R., Tarek, C., Hassane, E., Noureddine, E. H., & Khadija, O. (2021). Chemical proprieties of biopolymers (chitin/chitosan) and their synergic effects with endophytic Bacillus species: Unlimited applications in agriculture. Molecules, 26(4):1-26.
- Anas, A., Philip, R., & Singh, I. S. B. (2008). Chitosan as a wall material for a microencapsulated delivery system for Macrobrachium rosenbergii (de Man) larvae. Aquaculture Research, 39(8):885-890.
- Azmin, N., Nasir, M., & Hartati. (2019). Utilization of shrimp shell (Penaeus modonon) for making chitosan as a natural meat preservative. Oryza Jurnal Pendidikan Biologi, 8(1):9-15.
- Bastiaens, L., Soetemans, L., D’Hondt, E., & Elst, K. (2020). Sources of chitin and chitosan and their isolation. In L. A. M. V. D. Broek & C. G. Boeriu (Eds.), Chitin and chitosan: Properties and applications. (pp. 1-28). UK: John Wiley & Sons.
- Betchem, G., Johnson, N. A. N., & Wang, Y. (2019). The application of chitosan in the control of post-harvest diseases: A review. Journal of Plant Diseases and Protection, 126(6):495-507.
- Bolat, Y., Bilgin, S., Gunlu, A., Izci, L., Koca, S. B., Cetinkaya, S., & Koca, H. U. (2010). Chitin-chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pakistan Veterinary Journal, 30(4):227-231.
- Brito, D. Q., Santos, L. H. G., Passos, C. J. S., & Oliveira-Filho, E. C. (2021). Short‐term effects of wildfire ash on water quality parameters: A laboratory approach. Bulletin of Environmental Contamination and Toxicology, 107(3):500-505.
- Cha, S.-H., Lee, J.-S., Song, C.-B., Lee, K.-J., & Jeon, Y.-J. (2008). Effects of chitosan-coated diet on improving water quality and innate immunity in the olive flounder, Paralichthys olivaceus. Aquaculture, 278(1):110-118.
- Chaudhari, A. K., Das, S., Dwivedi, A., & Dubey, N. K. (2023). Application of chitosan and other biopolymers based edible coatings containing essential oils as green and innovative strategy for preservation of perishable food products: A review. International Journal of Biological Macromolecules, 253(8):127688-127688.
- Chen, G., Yin, B., Liu, H., Tan, B., Dong, X., Yang, Q., Chi, S., & Zhang, S. (2021). Supplementing chitosan oligosaccharide positively affects hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed dietary fish meal replacement with cottonseed protein concentrate: Effects on growth, gut microbiota, antioxidant function and immune response. Frontiers in Marine Science, 8(1):1-18.
- Chouhan, D., & Mandal, P. (2021). Applications of chitosan and chitosan based metallic nanoparticles in agrosciences-a review. International Journal of Biological Macromolecules, 166(1):1554-1569.
- Chouljenko, A., Mirtalebi, S., Hopper, S., Santos, F., Bolton, G., Bailey, C., & Christyn, B. (2024). Combining fish and crustacean byproducts as primary ingredients in pelleted aquafeed: The effect of byproduct type on pellet physical properties. Aquaculture Research, 2024(1):1-10.
- Confederat, L. G., Tuchilus, C. G., Dragan, M., Sha'at, M., & Dragostin, O. M. (2021). Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules, 26(12):1-17.
- Cui, J., Yu, Z., & Lau, D. (2016). Effect of acetyl group on mechanical properties of chitin/chitosan nanocrystal: A molecular dynamics study. International Journal of Molecular Sciences, 17(1):61-61.
- Díaz‐Montes, E., & Castro‐Muñoz, R. (2021). Trends in chitosan as a primary biopolymer for functional films and coatings manufacture for food and natural products. Polymers, 13(5):1-28.
- Divya, K., Smitha, V., & Jisha, M. S. (2018). Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. International Journal of Biological Macromolecules, 15(114):572-577.
- Ekwomadu, T. I., & Mwanza, M. (2023). Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: a review of the latest research. Agriculture, 13(9):1-20.
- El-Araby, A., El Ghadraoui, L., & Errachidi, F. (2022). Usage of biological chitosan against the contamination of post-harvest treatment of strawberries by Aspergillus niger. Frontiers in Sustainable Food Systems, 6(1):1-15.
- Elbahnaswy, S., Elshopakey, G. E., Ibrahim, I., & Habotta, O. A. (2021). Potential role of dietary chitosan nanoparticles against immunosuppression, inflammation, oxidative stress, and histopathological alterations induced by Pendimethalin toxicity in Nile tilapia. Fish & Shellfish Immunology, 118(1):270-282.
- Fadl, S. E., El‐Gammal, G. A., Abdo, W. S., Barakat, M., Sakr, O. A., Nassef, EEkwomadu, T. I., & Mwanza, M. (2023). Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: A review of the latest research. Agriculture, 13(9):1-20.
- Gad, D. M., & El‐Sheshtawy, H. S. (2020). Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae Infection. Aquaculture Research, 51(3):1120-1132.
- Gamil, Y., Hamed, M. G., Elsayed, M., Essawy, A., Medhat, S., Zayed, S. O., & Ismail, R. M. (2024). The anti-fungal effect of miconazole and miconazole-loaded chitosan nanoparticles gels in diabetic patients with oral candidiasis-randomized control clinical trial and microbiological analysis. BMC Oral Health, 24(1):196-196.
- Gharaie, S. S., Habibi, S., & Nazockdast, H. (2018). Fabrication and characterization of chitosan/gelatin/thermoplastic polyurethane blend nanofibers. Journal of Textiles and Fibrous Materials, 1(1):1-8.
- Gong, W., Sun, Y., Tu, T., Huang, J., Zhu, C., Zhang, J., Salah, M., Zhao, L., Xia, X., & Wang, Y. (2024). Chitosan inhibits Penicillium expansum possibly by binding to DNA and triggering apoptosis. International Journal of Biological Molecule, 259(1):1-9.
- Gowda, S., & Sriram, S. (2023). Green synthesis of chitosan silver nanocomposites and their antifungal activity against Colletotrichum truncatum causing anthracnose in chillies. Plant Nano Biology, 5(3):1-11.
- Goy, R. C., De Britto, D., & Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros, Ciência e Tecnologia, 19(3):241-247.
- Guo, H., Qiao, B., Ji, X., Wang, X., & Zhu, E. (2020). Antifungal activity and possible mechanisms of submicron chitosan dispersions against Alteraria alternata. Postharvest Biology and Technology, 161(3):1-8.
- Hazeena, S. H., Hou, C. Y., Zeng, J. H., Li, B. H., Lin, T. C., Liu, C. S., Chang, C. I., Hsieh, S. L., & Shih, M. K. (2022). Extraction optimization and structural characteristics of chitosan from cuttlefish (S. pharaonis sp.) bone. Materials, 15(22):1-15.
- Hsu, C.-Y., Ajaj, Y., Mahmoud, Z. H., Ghadir, G. K., Alani, Z. K., Hussein, M. M., Hussein, S. A., Karim, M. M., Al-Khalidi, A., Abbas, J. K., Kareem, A. H., & Kianfar, E. (2024). Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results in Chemistry, 7(1):1-24.
- Ibrahim, D., Neamat-Allah, A. N. F., Ibrahim, S. M., Eissa, H. M., Fawzey, M. M., Mostafa, D. I. A., El-Kader, S. A. A., Khater, S. I., & Khater, S. I. (2021). Dual effect of selenium loaded chitosan nanoparticles on growth, antioxidant, immune related genes expression, transcriptomics modulation of caspase 1, cytochrome P450 and heat shock protein and Aeromonas hydrophila resistance of Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 110(1):91-99.
- Ihsan, M., Priyambodo, B., & Muliasari, H. (2020). Training on making gel feed based on local ingredients as alternative feed for lobster cultivation on Lombok Island. Transformasi Jurnal Pengabdian Masyarakat, 16(1):1-11.
- Jin, Q., Yu, H., Wang, X., Li, K., & Li, P. (2017). Effect of the molecular weight of water-soluble chitosan on its fat-/cholesterol-binding capacities and inhibitory activities to pancreatic lipase. PeerJ, 5(1):1-22.
- Jones, C. M., Anh, T. L., & Priyambodo, B. (2019). Lobster aquaculture development in Vietnam and Indonesia. In E. V. Radhakrisnan, B. F. Phillips, & G. Achamveetil (Eds.), Lobsters: Biology, fisheries and aquaculture. Singapore: Springer Nature Singapore Pte.Ltd.
- Karamchandani, B. M., Chakraborty, S., Dalvi, S. G., & Satpute, S. K. (2022). Chitosan and its derivatives: Promising biomaterial in averting fungal diseases of sugarcane and other crops. Journal of Basic Microbiology, 62(5):533-554.
- Ke, C.-L., Deng, F.-S., Chuang, C.-Y., & Lin, C.-H. (2021). Antimicrobial actions and applications of chitosan. Polymers, 13(6):1-21.
- Ke, Y., Ding, B., Zhang, M., Dong, T., Fu, Y., Lv, Q., Ding, W., & Wang, X. (2022). Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus flavus and Aspergillus fumigatus. Carbohydrate Polymers, 275(1):1-8.
- Kumar, B. T. N., Thakur, N., Sharma, C., Shanthanagouda, A. H., Taygi, A., & Singh, A. (2022). Effect of dietary chitosan nanoparticles on immune response and disease resistance against Aeromonas hydrophila infection in tropical herbivore fish (Rohu, Labeo rohita). Aquaculture International, 30(5):2439-2452.
- Kurniawidi, D. W., Alaa, S., Nurhaliza, E., Safitri, D. O., Rahayu, S., Ali, M., & Amin, M. (2022). Synthesis and characterization of nano chitosan from vannamei shrimp shell (Litopenaeus vannamei). Jurnal Ilmiah Perikanan dan Kelautan, 14(2):380-387.
- Leslie, J. F., & Summerell, B. A. (2006). Fusarium laboratory manual (1st ed.). Blackwell Pub.
- Li, J., & Zhuang, S. (2020). Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. European Polymer Journal, 138(1):1-12.
- Liu, J., Xu, W., Liu, Y., Wang, Y., Zhang, J., Wang, Z., Mai, K., & Ai, Q. (2022). Effects of chitosan-coated microdiet on dietary physical properties, growth performance, digestive enzyme activities, antioxidant capacity, and inflammation response of large yellow croaker (Larimichthys crocea) larvae. Aquaculture Nutrition, 2022(1):1-11.
- Lübeck, M., & Lübeck, P. S. (2022). Fungal cell factories for efficient and sustainable production of proteins and peptides. Microorganisms, 10(4):1-24.
- Luthfiyana, N., Bija, S., Nugraeni, C. D., Lembang, M. S., Anwar, E., Laksmitawati, D. R., & Ratrinia, P. W. (2022). Characteristics and antibacterial activity of chitosan nanoparticles from mangrove crab shell (Scylla sp.) in Tarakan Waters, North Kalimantan, Indonesia. Biodiversitas, 23(8):4018-4025.
- Masruriati, E., Ariyanti, Imadahidayah, T., & Sulistianingsih, E. N. (2020). Utilization of chitosan from feather clam shells (Anadara antiquate) as a preservative for stingrays (Dasyatis sp.) and white shrimp (Litopenaeus vannamei). Riset Informasi Kesehatan, 9(1):12-21.
- Mathaba, M., & Daramola, M. O. (2020). Effect of chitosan's degree of deacetylation on the performance of PES membrane infused with chitosan during AMD treatment. Membranes, 10(3):1-16.
- Metin, C., Alparslan, Y., Baygar, T., & Baygar, T. (2019). Physicochemical, microstructural and thermal characterization of chitosan from blue crab shell waste and its bioactivity characteristics. Journal of Polymers and the Environment, 27(11):2552-2561.
- Naiel, M. A. E., Ismael, N. E. M., Abd El-hameed, S. A. A., & Amer, M. S. (2020). The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture, 523(1):1-11.
- Nugroho, I. L., Masithah, E. D., & Pursetyo, K. T. (2020). The effect of hydrochloric acid concentration and temperature demineralization on characteristics of chitin from penshell (Atrina pectinata). IOP Conference Series. Earth and Environmental Science, 441(012152):1-7.
- Onwuka, J. C., Agbaji, E. B., Ajibola, V. O., & Okibe, F. G. (2019). Thermodynamic pathway of lignocellulosic acetylation process. BMC Chemistry, 13(1):1-11.
- Picos-Corrales, L. A., Morales-Burgos, A. M., Ruelas-Leyva, J. P., Crini, G., García-Armenta, E., Jimenez-Lam, S. A., Ayón-Reyna, L. E., Rocha-Alonzo, F., Calderón-Zamora, L., Osuna-Martínez, U., Calderón-Castro, A., De-Paz-Arroyo, G., & Inzunza-Camacho, L. N. (2023). Chitosan as an outstanding polysaccharide improving health-commodities of humans and environmental protection. Polymers, 15(3):1-27.
- Román-Doval, R., Torres-Arellanes, S. P., Tenorio-Barajas, A. Y., Gómez-Sánchez, A., & Valencia-Lazcano, A. A. (2023). Chitosan: Properties and its application in agriculture in context of molecular weight. Polymers, 15(13):1-26.
- Romanazzi, G., Feliziani, E., & Sivakumar, D. (2018). Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Frontiers in Microbiology, 9(1):1-9.
- Romanazzi, G., & Moumni, M. (2022). Chitosan and other edible coatings to extend shelf life, manage postharvest decay, and reduce loss and waste of fresh fruits and vegetables. Current Opinion in Biotechnology, 78(1):1-9.
- Salam, M. A., Rahman, M. A., Paul, S. I., Islam, F., Barman, A. K., Rahman, Z., Shaha, D. C., Rahman, M. M., & Islam, T. (2021). Dietary chitosan promotes the growth, biochemical composition, gut microbiota, hematological parameters and internal organ morphology of juvenile Barbonymus gonionotus. PloS One, 16(11):1-23.
- Saputra, D., Ula, F. R., Fadhila, A. B. N., Sijabat, Y. Y., Romadoni, A. A., & Windarto, S. (2022). Nano-chitosan spray as a preservative and food security of fishery products in the middle of the Covid-19 Pandemic. Jurnal Ilmiah Perikanan dan Kelautan, 14(1):71-82.
- Shahbaz, U., Basharat, S., Javed, U., Bibi, A., & Yu, X. B. (2023). Chitosan: A multipurpose polymer in food industry. Polymer Bulletin, 80(4):3547-3569.
- Shi, F., Qiu, X., Nie, L., Hu, L., Babu V, S., Lin, Q., Zhang, Y., Chen, L., Li, J., Lin, L., & Qin, Z. (2020). Effects of oligochitosan on the growth, immune responses and gut microbes of tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 106(1):563-573.
- Shrestha, R., Thenissery, A., Khupse, R., & Rajashekara, G. (2023). Strategies for the preparation of chitosan derivatives for antimicrobial, drug delivery, and agricultural applications: A review. Molecules, 28(22):1-39.
- Singh, T., Vesentini, D., Singh, A. P., & Daniel, G. (2008). Effect of chitosan on physiological, morphological, and ultrastructural characteristics of wood-degrading fungi. International Biodeterioration & Biodegradation, 62(2):116-124.
- Statista. (2024). Volume of lobsters exported from Indonesia from 2014 to 2022.
- Teixeira-Santos, R., Lima, M., Gomes, L. C., & Mergulhão, F. J. (2021). Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience, 24(12):1-23.
- Volpe, M. G., Varricchio, E., Coccia, E., Santagata, G., Di Stasio, M., Malinconico, M., & Paolucci, M. (2012). Manufacturing pellets with different binders: effect on water stability and feeding response in juvenile Cherax albidus. Aquaculture, 324(12):104-110.
- Wang, R., Li, X., Tang, J., Liu, J., Yin, H., Li, R., & Ye, S. (2023). Dietary effect chitosan nanoparticles on growth performance, immunity and resistance against Vibrio splendidus in the sea cucumber Apostichopus japonicas. Aquaculture Reports, 30(1):1-7.
- Wu, J., Chang, J., Liu, J., Huang, J., Song, Z., Xie, X., Wei, L., Xu, J., Huang, S., Cheng, D., Li, Y., Xu, H., & Zhang, Z. (2023). Chitosan-based nanopesticides enhanced anti-fungal activity against strawberry anthracnose as “sugar-coated bombs”. International Journal of Biological Macromolecules, 253(36):126947-126947.
- Xu, H., Wang, X., Liang, Q., Xu, R., Liu, J., & Yu, D. (2023). Dietary chitosan moderates the growth rate, antioxidant activity, immunity, intestinal morphology and resistance against Aeromonas hydrophila of juvenile hybrid sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂). International Journal of Biological Macromolecules, 224(1):1012-1024.
- Younes, I., & Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. structure, properties and applications. Marine Drugs, 13(3):1133-1174.
- Zeng, A., Wang, Y., Li, D., Guo, J., & Chen, Q. (2021). Preparation and antibacterial properties of polycaprolactone/quaternized chitosan blends. Chinese Journal of Chemical Engineering, 32(4):462-471.
References
Abdel-Razek, N. (2019). Antimicrobial activities of chitosan nanoparticles against pathogenic microorganisms in Nile tilapia, Oreochromis niloticus. Aquaculture International, 27(5):1315-1330.
Adamczuk, A., Kercheva, M., Hristova, M., & Jozefaciuk, G. (2021). Impact of chitosan on water stability and wettability of soils. Materials, 14(24):1-12.
Ahyat, N. M., Mohamad, F., Ahmad, A., & Azmi, A. A. (2017). Chitin and chitosan extraction from Portunis pelagicus. Malaysian Journal of Analytical Sciences, 21(4):770-777.
Aly, S. M., Eissa, A. E., Abdel-Razek, N., & El-Ramlawy, A. O. (2023). The antibacterial activity and immunomodulatory effect of naturally synthesized chitosan and silver nanoparticles against Pseudomonas fluorescence infection in Nile tilapia (Oreochromis niloticus): an in vivo study. Fish & Shellfish Immunology, 135(1):108628-108628.
Amine, R., Tarek, C., Hassane, E., Noureddine, E. H., & Khadija, O. (2021). Chemical proprieties of biopolymers (chitin/chitosan) and their synergic effects with endophytic Bacillus species: Unlimited applications in agriculture. Molecules, 26(4):1-26.
Anas, A., Philip, R., & Singh, I. S. B. (2008). Chitosan as a wall material for a microencapsulated delivery system for Macrobrachium rosenbergii (de Man) larvae. Aquaculture Research, 39(8):885-890.
Azmin, N., Nasir, M., & Hartati. (2019). Utilization of shrimp shell (Penaeus modonon) for making chitosan as a natural meat preservative. Oryza Jurnal Pendidikan Biologi, 8(1):9-15.
Bastiaens, L., Soetemans, L., D’Hondt, E., & Elst, K. (2020). Sources of chitin and chitosan and their isolation. In L. A. M. V. D. Broek & C. G. Boeriu (Eds.), Chitin and chitosan: Properties and applications. (pp. 1-28). UK: John Wiley & Sons.
Betchem, G., Johnson, N. A. N., & Wang, Y. (2019). The application of chitosan in the control of post-harvest diseases: A review. Journal of Plant Diseases and Protection, 126(6):495-507.
Bolat, Y., Bilgin, S., Gunlu, A., Izci, L., Koca, S. B., Cetinkaya, S., & Koca, H. U. (2010). Chitin-chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pakistan Veterinary Journal, 30(4):227-231.
Brito, D. Q., Santos, L. H. G., Passos, C. J. S., & Oliveira-Filho, E. C. (2021). Short‐term effects of wildfire ash on water quality parameters: A laboratory approach. Bulletin of Environmental Contamination and Toxicology, 107(3):500-505.
Cha, S.-H., Lee, J.-S., Song, C.-B., Lee, K.-J., & Jeon, Y.-J. (2008). Effects of chitosan-coated diet on improving water quality and innate immunity in the olive flounder, Paralichthys olivaceus. Aquaculture, 278(1):110-118.
Chaudhari, A. K., Das, S., Dwivedi, A., & Dubey, N. K. (2023). Application of chitosan and other biopolymers based edible coatings containing essential oils as green and innovative strategy for preservation of perishable food products: A review. International Journal of Biological Macromolecules, 253(8):127688-127688.
Chen, G., Yin, B., Liu, H., Tan, B., Dong, X., Yang, Q., Chi, S., & Zhang, S. (2021). Supplementing chitosan oligosaccharide positively affects hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed dietary fish meal replacement with cottonseed protein concentrate: Effects on growth, gut microbiota, antioxidant function and immune response. Frontiers in Marine Science, 8(1):1-18.
Chouhan, D., & Mandal, P. (2021). Applications of chitosan and chitosan based metallic nanoparticles in agrosciences-a review. International Journal of Biological Macromolecules, 166(1):1554-1569.
Chouljenko, A., Mirtalebi, S., Hopper, S., Santos, F., Bolton, G., Bailey, C., & Christyn, B. (2024). Combining fish and crustacean byproducts as primary ingredients in pelleted aquafeed: The effect of byproduct type on pellet physical properties. Aquaculture Research, 2024(1):1-10.
Confederat, L. G., Tuchilus, C. G., Dragan, M., Sha'at, M., & Dragostin, O. M. (2021). Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules, 26(12):1-17.
Cui, J., Yu, Z., & Lau, D. (2016). Effect of acetyl group on mechanical properties of chitin/chitosan nanocrystal: A molecular dynamics study. International Journal of Molecular Sciences, 17(1):61-61.
Díaz‐Montes, E., & Castro‐Muñoz, R. (2021). Trends in chitosan as a primary biopolymer for functional films and coatings manufacture for food and natural products. Polymers, 13(5):1-28.
Divya, K., Smitha, V., & Jisha, M. S. (2018). Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. International Journal of Biological Macromolecules, 15(114):572-577.
Ekwomadu, T. I., & Mwanza, M. (2023). Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: a review of the latest research. Agriculture, 13(9):1-20.
El-Araby, A., El Ghadraoui, L., & Errachidi, F. (2022). Usage of biological chitosan against the contamination of post-harvest treatment of strawberries by Aspergillus niger. Frontiers in Sustainable Food Systems, 6(1):1-15.
Elbahnaswy, S., Elshopakey, G. E., Ibrahim, I., & Habotta, O. A. (2021). Potential role of dietary chitosan nanoparticles against immunosuppression, inflammation, oxidative stress, and histopathological alterations induced by Pendimethalin toxicity in Nile tilapia. Fish & Shellfish Immunology, 118(1):270-282.
Fadl, S. E., El‐Gammal, G. A., Abdo, W. S., Barakat, M., Sakr, O. A., Nassef, EEkwomadu, T. I., & Mwanza, M. (2023). Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: A review of the latest research. Agriculture, 13(9):1-20.
Gad, D. M., & El‐Sheshtawy, H. S. (2020). Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae Infection. Aquaculture Research, 51(3):1120-1132.
Gamil, Y., Hamed, M. G., Elsayed, M., Essawy, A., Medhat, S., Zayed, S. O., & Ismail, R. M. (2024). The anti-fungal effect of miconazole and miconazole-loaded chitosan nanoparticles gels in diabetic patients with oral candidiasis-randomized control clinical trial and microbiological analysis. BMC Oral Health, 24(1):196-196.
Gharaie, S. S., Habibi, S., & Nazockdast, H. (2018). Fabrication and characterization of chitosan/gelatin/thermoplastic polyurethane blend nanofibers. Journal of Textiles and Fibrous Materials, 1(1):1-8.
Gong, W., Sun, Y., Tu, T., Huang, J., Zhu, C., Zhang, J., Salah, M., Zhao, L., Xia, X., & Wang, Y. (2024). Chitosan inhibits Penicillium expansum possibly by binding to DNA and triggering apoptosis. International Journal of Biological Molecule, 259(1):1-9.
Gowda, S., & Sriram, S. (2023). Green synthesis of chitosan silver nanocomposites and their antifungal activity against Colletotrichum truncatum causing anthracnose in chillies. Plant Nano Biology, 5(3):1-11.
Goy, R. C., De Britto, D., & Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros, Ciência e Tecnologia, 19(3):241-247.
Guo, H., Qiao, B., Ji, X., Wang, X., & Zhu, E. (2020). Antifungal activity and possible mechanisms of submicron chitosan dispersions against Alteraria alternata. Postharvest Biology and Technology, 161(3):1-8.
Hazeena, S. H., Hou, C. Y., Zeng, J. H., Li, B. H., Lin, T. C., Liu, C. S., Chang, C. I., Hsieh, S. L., & Shih, M. K. (2022). Extraction optimization and structural characteristics of chitosan from cuttlefish (S. pharaonis sp.) bone. Materials, 15(22):1-15.
Hsu, C.-Y., Ajaj, Y., Mahmoud, Z. H., Ghadir, G. K., Alani, Z. K., Hussein, M. M., Hussein, S. A., Karim, M. M., Al-Khalidi, A., Abbas, J. K., Kareem, A. H., & Kianfar, E. (2024). Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results in Chemistry, 7(1):1-24.
Ibrahim, D., Neamat-Allah, A. N. F., Ibrahim, S. M., Eissa, H. M., Fawzey, M. M., Mostafa, D. I. A., El-Kader, S. A. A., Khater, S. I., & Khater, S. I. (2021). Dual effect of selenium loaded chitosan nanoparticles on growth, antioxidant, immune related genes expression, transcriptomics modulation of caspase 1, cytochrome P450 and heat shock protein and Aeromonas hydrophila resistance of Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 110(1):91-99.
Ihsan, M., Priyambodo, B., & Muliasari, H. (2020). Training on making gel feed based on local ingredients as alternative feed for lobster cultivation on Lombok Island. Transformasi Jurnal Pengabdian Masyarakat, 16(1):1-11.
Jin, Q., Yu, H., Wang, X., Li, K., & Li, P. (2017). Effect of the molecular weight of water-soluble chitosan on its fat-/cholesterol-binding capacities and inhibitory activities to pancreatic lipase. PeerJ, 5(1):1-22.
Jones, C. M., Anh, T. L., & Priyambodo, B. (2019). Lobster aquaculture development in Vietnam and Indonesia. In E. V. Radhakrisnan, B. F. Phillips, & G. Achamveetil (Eds.), Lobsters: Biology, fisheries and aquaculture. Singapore: Springer Nature Singapore Pte.Ltd.
Karamchandani, B. M., Chakraborty, S., Dalvi, S. G., & Satpute, S. K. (2022). Chitosan and its derivatives: Promising biomaterial in averting fungal diseases of sugarcane and other crops. Journal of Basic Microbiology, 62(5):533-554.
Ke, C.-L., Deng, F.-S., Chuang, C.-Y., & Lin, C.-H. (2021). Antimicrobial actions and applications of chitosan. Polymers, 13(6):1-21.
Ke, Y., Ding, B., Zhang, M., Dong, T., Fu, Y., Lv, Q., Ding, W., & Wang, X. (2022). Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus flavus and Aspergillus fumigatus. Carbohydrate Polymers, 275(1):1-8.
Kumar, B. T. N., Thakur, N., Sharma, C., Shanthanagouda, A. H., Taygi, A., & Singh, A. (2022). Effect of dietary chitosan nanoparticles on immune response and disease resistance against Aeromonas hydrophila infection in tropical herbivore fish (Rohu, Labeo rohita). Aquaculture International, 30(5):2439-2452.
Kurniawidi, D. W., Alaa, S., Nurhaliza, E., Safitri, D. O., Rahayu, S., Ali, M., & Amin, M. (2022). Synthesis and characterization of nano chitosan from vannamei shrimp shell (Litopenaeus vannamei). Jurnal Ilmiah Perikanan dan Kelautan, 14(2):380-387.
Leslie, J. F., & Summerell, B. A. (2006). Fusarium laboratory manual (1st ed.). Blackwell Pub.
Li, J., & Zhuang, S. (2020). Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. European Polymer Journal, 138(1):1-12.
Liu, J., Xu, W., Liu, Y., Wang, Y., Zhang, J., Wang, Z., Mai, K., & Ai, Q. (2022). Effects of chitosan-coated microdiet on dietary physical properties, growth performance, digestive enzyme activities, antioxidant capacity, and inflammation response of large yellow croaker (Larimichthys crocea) larvae. Aquaculture Nutrition, 2022(1):1-11.
Lübeck, M., & Lübeck, P. S. (2022). Fungal cell factories for efficient and sustainable production of proteins and peptides. Microorganisms, 10(4):1-24.
Luthfiyana, N., Bija, S., Nugraeni, C. D., Lembang, M. S., Anwar, E., Laksmitawati, D. R., & Ratrinia, P. W. (2022). Characteristics and antibacterial activity of chitosan nanoparticles from mangrove crab shell (Scylla sp.) in Tarakan Waters, North Kalimantan, Indonesia. Biodiversitas, 23(8):4018-4025.
Masruriati, E., Ariyanti, Imadahidayah, T., & Sulistianingsih, E. N. (2020). Utilization of chitosan from feather clam shells (Anadara antiquate) as a preservative for stingrays (Dasyatis sp.) and white shrimp (Litopenaeus vannamei). Riset Informasi Kesehatan, 9(1):12-21.
Mathaba, M., & Daramola, M. O. (2020). Effect of chitosan's degree of deacetylation on the performance of PES membrane infused with chitosan during AMD treatment. Membranes, 10(3):1-16.
Metin, C., Alparslan, Y., Baygar, T., & Baygar, T. (2019). Physicochemical, microstructural and thermal characterization of chitosan from blue crab shell waste and its bioactivity characteristics. Journal of Polymers and the Environment, 27(11):2552-2561.
Naiel, M. A. E., Ismael, N. E. M., Abd El-hameed, S. A. A., & Amer, M. S. (2020). The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture, 523(1):1-11.
Nugroho, I. L., Masithah, E. D., & Pursetyo, K. T. (2020). The effect of hydrochloric acid concentration and temperature demineralization on characteristics of chitin from penshell (Atrina pectinata). IOP Conference Series. Earth and Environmental Science, 441(012152):1-7.
Onwuka, J. C., Agbaji, E. B., Ajibola, V. O., & Okibe, F. G. (2019). Thermodynamic pathway of lignocellulosic acetylation process. BMC Chemistry, 13(1):1-11.
Picos-Corrales, L. A., Morales-Burgos, A. M., Ruelas-Leyva, J. P., Crini, G., García-Armenta, E., Jimenez-Lam, S. A., Ayón-Reyna, L. E., Rocha-Alonzo, F., Calderón-Zamora, L., Osuna-Martínez, U., Calderón-Castro, A., De-Paz-Arroyo, G., & Inzunza-Camacho, L. N. (2023). Chitosan as an outstanding polysaccharide improving health-commodities of humans and environmental protection. Polymers, 15(3):1-27.
Román-Doval, R., Torres-Arellanes, S. P., Tenorio-Barajas, A. Y., Gómez-Sánchez, A., & Valencia-Lazcano, A. A. (2023). Chitosan: Properties and its application in agriculture in context of molecular weight. Polymers, 15(13):1-26.
Romanazzi, G., Feliziani, E., & Sivakumar, D. (2018). Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Frontiers in Microbiology, 9(1):1-9.
Romanazzi, G., & Moumni, M. (2022). Chitosan and other edible coatings to extend shelf life, manage postharvest decay, and reduce loss and waste of fresh fruits and vegetables. Current Opinion in Biotechnology, 78(1):1-9.
Salam, M. A., Rahman, M. A., Paul, S. I., Islam, F., Barman, A. K., Rahman, Z., Shaha, D. C., Rahman, M. M., & Islam, T. (2021). Dietary chitosan promotes the growth, biochemical composition, gut microbiota, hematological parameters and internal organ morphology of juvenile Barbonymus gonionotus. PloS One, 16(11):1-23.
Saputra, D., Ula, F. R., Fadhila, A. B. N., Sijabat, Y. Y., Romadoni, A. A., & Windarto, S. (2022). Nano-chitosan spray as a preservative and food security of fishery products in the middle of the Covid-19 Pandemic. Jurnal Ilmiah Perikanan dan Kelautan, 14(1):71-82.
Shahbaz, U., Basharat, S., Javed, U., Bibi, A., & Yu, X. B. (2023). Chitosan: A multipurpose polymer in food industry. Polymer Bulletin, 80(4):3547-3569.
Shi, F., Qiu, X., Nie, L., Hu, L., Babu V, S., Lin, Q., Zhang, Y., Chen, L., Li, J., Lin, L., & Qin, Z. (2020). Effects of oligochitosan on the growth, immune responses and gut microbes of tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 106(1):563-573.
Shrestha, R., Thenissery, A., Khupse, R., & Rajashekara, G. (2023). Strategies for the preparation of chitosan derivatives for antimicrobial, drug delivery, and agricultural applications: A review. Molecules, 28(22):1-39.
Singh, T., Vesentini, D., Singh, A. P., & Daniel, G. (2008). Effect of chitosan on physiological, morphological, and ultrastructural characteristics of wood-degrading fungi. International Biodeterioration & Biodegradation, 62(2):116-124.
Statista. (2024). Volume of lobsters exported from Indonesia from 2014 to 2022.
Teixeira-Santos, R., Lima, M., Gomes, L. C., & Mergulhão, F. J. (2021). Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience, 24(12):1-23.
Volpe, M. G., Varricchio, E., Coccia, E., Santagata, G., Di Stasio, M., Malinconico, M., & Paolucci, M. (2012). Manufacturing pellets with different binders: effect on water stability and feeding response in juvenile Cherax albidus. Aquaculture, 324(12):104-110.
Wang, R., Li, X., Tang, J., Liu, J., Yin, H., Li, R., & Ye, S. (2023). Dietary effect chitosan nanoparticles on growth performance, immunity and resistance against Vibrio splendidus in the sea cucumber Apostichopus japonicas. Aquaculture Reports, 30(1):1-7.
Wu, J., Chang, J., Liu, J., Huang, J., Song, Z., Xie, X., Wei, L., Xu, J., Huang, S., Cheng, D., Li, Y., Xu, H., & Zhang, Z. (2023). Chitosan-based nanopesticides enhanced anti-fungal activity against strawberry anthracnose as “sugar-coated bombs”. International Journal of Biological Macromolecules, 253(36):126947-126947.
Xu, H., Wang, X., Liang, Q., Xu, R., Liu, J., & Yu, D. (2023). Dietary chitosan moderates the growth rate, antioxidant activity, immunity, intestinal morphology and resistance against Aeromonas hydrophila of juvenile hybrid sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂). International Journal of Biological Macromolecules, 224(1):1012-1024.
Younes, I., & Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. structure, properties and applications. Marine Drugs, 13(3):1133-1174.
Zeng, A., Wang, Y., Li, D., Guo, J., & Chen, Q. (2021). Preparation and antibacterial properties of polycaprolactone/quaternized chitosan blends. Chinese Journal of Chemical Engineering, 32(4):462-471.