Date Log
Copyright (c) 2025 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
Environmental Effect on the Growth and Enzyme Activity of Fucoidanase-Producing Bacteria Cytobacillus kochii GSD
Corresponding Author(s) : Agus Setyawan
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 17 No. 2 (2025): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
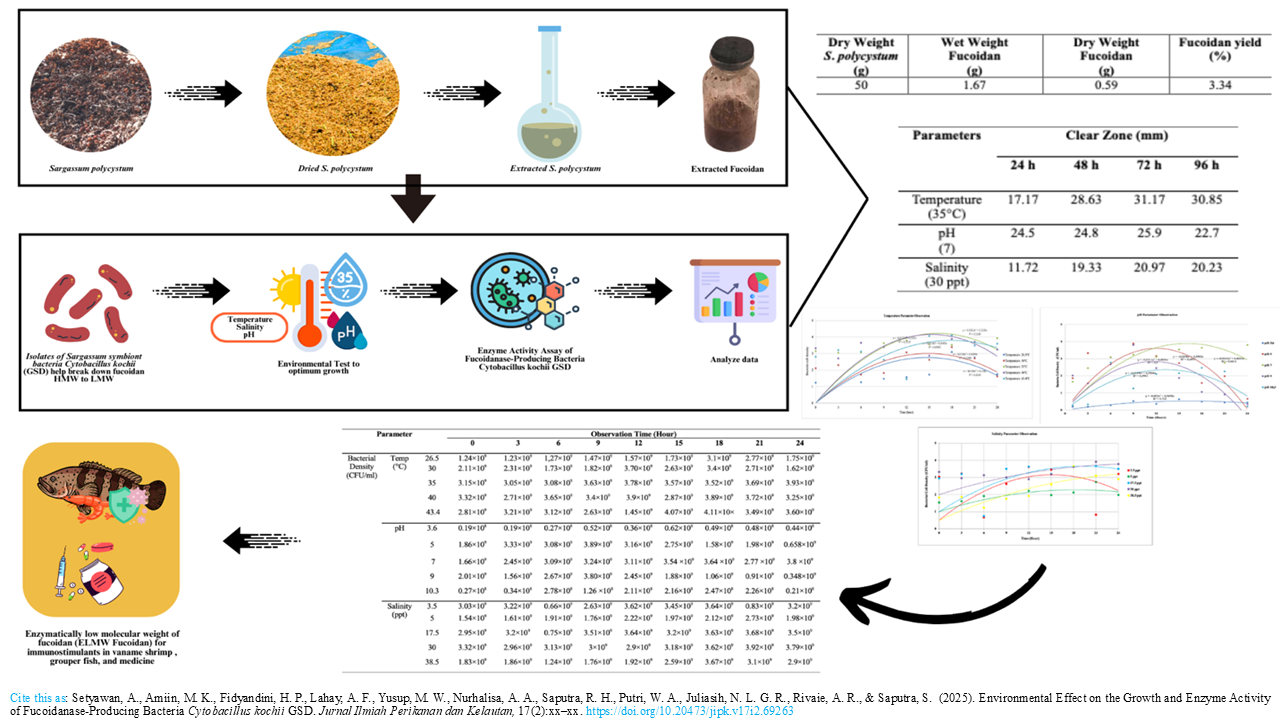
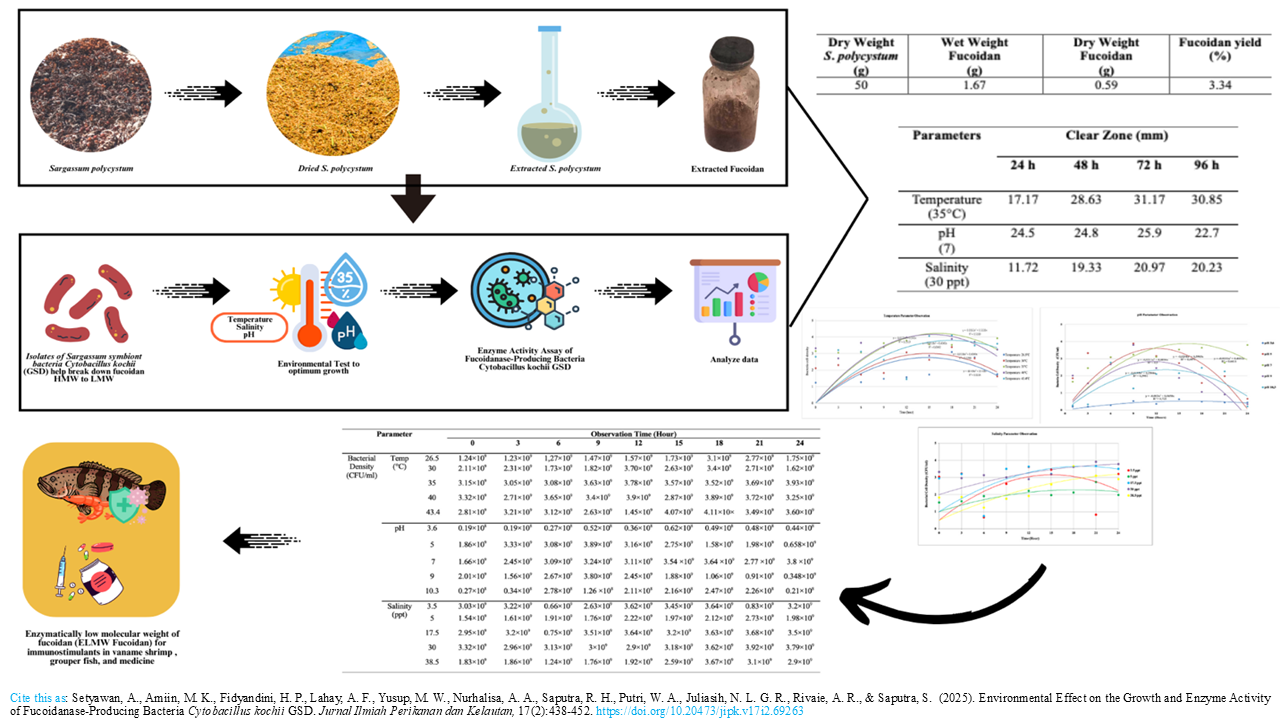
Graphical Abstract
Highlight Research
1. Sargassum polycystum has been extracted and analyzed.
2. Cytobacillus kochii GSD in response to fucoidanase-producing enzyme activity was analyzed.
3. Optimum temperature, pH, and salinity can suppress the activity of the fucoidanase enzyme through the inhibition test.
4. Cytobacillus kochii GSD can produce fucoidanase enzyme with low molecular weight.
Abstract
Extensive research has shown that low molecular weight fucoidan exhibits significantly greater biological activity than its high molecular weight. C. kochi GSD, a Sargassum symbiont bacterium, is proven to have the activity of hydrolyze fucoidan. This study proposes the growth optimization and fucoidanase enzymatic activity of C. kochii GSD bacteria under varying environmental conditions (temperature, pH, and salinity) cultured in basic liquid medium (BLM) for 48 hours. Based on Response Surface Methodology (RSM), the range of temperature, pH, and salinity for the growth optimization test of C. kochi GSD bacteria were 26.591, 30, 35, 40, and 43.49oC, the pH used starts from 3.636, 5, 7, 9, and 10.363, while the salinity to be used starts from 3.522, 5, 17.5, 30, and 38.522 ppt, respectively. The best conditions for growth of each environment were then continued with the test of fucoidanase enzyme activity in vitro. The results showed that C. kochii GSD bacteria grew optimally at temperature, pH, and salinity of 35oC, 7, and 30 ppt, respectively. The optimum enzyme activity of C. kochii GSD is at 72 hours with the forming of clear zones on media containing fucoidan and given Cetylpyridinium chloride (CPC) solution with clear zone diameters of 31.17 mm (temperature), 25.9 mm (pH), and 20.97 mm (salinity), respectively. The conclusion of this study is a high novelty finding to produce low molecular weight fucoidan enzymatically with C. kochii GSD bacteria to be used as an immunostimulant.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Abbas, M. F., Karim, D. K., Kareem, H. R., Kamil, M. M., Al-Musawi, M. H., Asker, M. H., Ghanami, M., Shahriari-Khalaji, M., Sattar, M., Mirhaj, M., Sharifianjazi, F., Tavamaishvili, K., Mohabbatkhah, M., Soheily, A., Noory, P., & Tavakoli, M. (2025). Fucoidan and its derivatives: From extraction to cutting-edge biomedical applications. Carbohydrate Polymers, 357(6):123468-123476.
- Abdel-Latif, H. M. R., Dawood, M. A. O., Alagawany, M., Faggio, C., Nowosad, J., & Kucharczyk, D. (2022). Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish and Shellfish Immunology, 122(3):115-130.
- Anisha, G. S., Padmakumari, S., Patel, A. K., Pandey, A., & Singhania, R. R. (2022). Fucoidan from marine macroalgae: Biological actions and applications in regenerative medicine, drug delivery systems and food industry. Journal Bioengineering, 9(9):1-28.
- Arisandi, A., Wardani, M. K., Badami, K., & Aranida, G. D. (2017). The impact of salinity differences on the viability of Vibrio fluvialis bacteria. Jurnal Ilmiah Perikanan Dan Kelautan, 9(2):91-97.
- Barzkar, N., Rungsardthong, V., Tamadoni Jahromi, S., Laraib, Q., Das, R., Babich, O., & Sukhikh, S. (2023). A recent update on fucoidonase: Source, isolation methods and its enzymatic activity. Frontiers in Marine Science, 10(5):1-13.
- Chen, S., & Li, D. (2023). Systematic and critical review on the antiviral bioactivities of complex carbohydrates against human noroviruses. Journal of Functional Foods, 108(9):1-8.
- Cholaraj, R., & Venkatachalam, R. (2024). Investigation of antioxidant and anticancer potential of fucoidan (in-vitro & in-silico) from brown seaweed Padina boergesenii. Algal Research, 79(5):103442-103450.
- Dumorné K, Córdova D. C, Astorga-Eló, M, & Renganathan, P. (2017). Extremozymes: A potential source for industrial applications. Journal of Microbiology and Biotechnology, 27(4):649-659.
- Elshazly, T. M., Salvatori, D., Elattar, H., Bourauel, C., & Keilig, L. (2023). Effect of trimming line design and edge extension of orthodontic aligners on force transmission: A 3D finite element study. Journal of the Mechanical Behavior of Biomedical Materials, 140(4):105741-105750.
- Fahmid, S., Jabeen, R., Mehar, S., Sajjad, N., Behlil, F., Riaz, M., Jameel, N., Ishtiyaq, H., Mukhtar, F., Khan, N., & Ali, J. (2023). Biomimetic synthesis and use of silver nanoparticles, an innocuous stratagem to combat fungal diseases in plants. Journal of Molecular Liquids, 391(12):123217-123225.
- Fernando, I. P. S., Dias, M. K. H. M., Madusanka, D. M. D., Han, E. J., Kim, M. J., Heo, S., Lee, K., Cheong, S. H., & Ahn, G. (2021). Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. International Journal of Biological Macromolecules, 168(1):620-630.
- Furqonita, A., Aritonang, A. B., & Wibowo, M. A. (2021). Synthesis of Bi3+ DOPED TiO2 and photocatalysis activity test of E. coli antibacteria under visible irradiation. Indonesian Journal of Pure and Applied Chemistry, 4(2):69-80.
- Hikariastri, P., Winarno, H., Kusmardi, K., Laksmitawati, D. R., & Abdillah, S. (2019). Anti-inflammatory activity of fucoidan crude extract from Sargassum crassifolium against LPS-induced RAW 264.7 cells. Jurnal Kefarmasian Indonesia, 9(2):97-105.
- Hu, S., Wang, J., Wang, J., Li, S., Jiang, W., & Liu, Y. (2017). Renoprotective effect of fucoidan from Acaudina molpadioides in streptozotocin/high fat diet-induced type 2 diabetic mice. Journal of Functional Foods, 31(4):123-130.
- Ikeda-Ohtsubo, W., López Nadal, A., Zaccaria, E., Iha, M., Kitazawa, H., Kleerebezem, M., & Brugman, S. (2020). Intestinal microbiota and immune modulation in zebrafish by fucoidan from Okinawa Mozuku (Cladosiphon okamuranus). Frontiers in Nutrition, 7(6):1-12.
- Kim, C., Alrefaei, R., Bushlaibi, M., Ndegwa, E., Kaseloo, P., & Wynn, C. (2019). Influence of growth temperature on thermal tolerance of leading foodborne pathogens. Food Science & Nutrition, 7(12):4027-4036.
- Kuddus, M. (2019). Introduction to food enzymes. In M. Kuddus (Ed.), Enzymes in food biotechnology. Production, applications, and future prospects. (pp. 1-18). Academic Press.
- Kusaykin, M. I., Silchenko, A. S., Zakharenko, A. M., & Zvyagintseva, T. N. (2016). Fucoidanases. Glycobiology, 26(1):3-12.
- Li, Q., Jiang, C., Tan, H., Zhao, X., Li, K., & Yin, H. (2021). Characterization of recombinant E. coli expressing a novel fucosidase from Bacillus cereus 2–8 belonging to GH95 family. Protein Expression and Purification, 186(12):105897-105904.
- Liu, S., Wang, Q., Shao, Z., Liu, Q., He, Y., Ren, D., Yang, H., & Li, X. (2023). Purification and characterization of the enzyme fucoidanase from Cobetia amphilecti utilizing fucoidan from Undaria pinnatifida. Foods, 12(7):1-17.
- Ma, Y., Zhang, L., Ma, X., Bai, K., Tian, Z., Wang, Z., Muratkhan, M., Wang, X., Lü, X., & Liu, M. (2024). Saccharide mapping as an extraordinary method on characterization and identification of plant and fungi polysaccharides: A review. International Journal of Biological Macromolecules, 275(08):133350-133367.
- Malyarenko, O. S., Malyarenko, T. V., Usoltseva, R. V., Silchenko, A. S., Kicha, A. A., Ivanchina, N. V., & Ermakova, S. P. (2021). Fucoidan from brown algae Fucus evanescens potentiates the anti-proliferative efficacy of asterosaponins from starfish Asteropsis carinifera in 2D and 3D models of melanoma cells. International Journal of Biological Macromolecules, 185(8):31-39.
- Manivasagan, P. & Oh, J. (2015). Production of a novel fucoidanase for the green synthesis of gold nanoparticles by Streptomyces sp. and its cytotoxic effect on hela cell. Marine Drugs. 13(11):6818-6837.
- Murtiyaningsih, H. & Hazmi, M. (2017). Isolation and cellulase enzyme activities assays in cellulolytic bacteria origin from soil waste. Agritrop – Journal of Agricultural Science. 15(2):293-308.
- Nagappan, H., Pee, P. P., Kee, S. H. Y., Ow, J. T., Yan, S. W., Chew, L. Y., & Kong, K. W. (2017). Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: Low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Research International, 27(1):950-958.
- Naoe, T., Hasebe, A., Horiuchi, R., Makita, Y., Okazaki, Y., Yasuda., Matsuo K., Yoshida, Y., Tsuga, K., Abe, Y & Yokoyama, A. (2020). Development of tissue conditioner containing cetylpyridinium chloride montmorillonite as new antimicrobial agent: Pilot study on antimicrobial activity and biocompatibility. Journal of Prosthodontic Research, 64(4):436-443.
- Panjaitan, R. S., & Natalia, L. (2021). Extraction of sulfate polysaccharide from Sargassum polycystum using microwave-assisted extraction method and its toxicity test. Jurnal Pascapanen Dan Bioteknologi Kelautan Dan Perikanan, 16(1):23-32.
- Pavão, M. S. G., & De Souza Cardoso, F. (2022). Seaweed fucoidans and their marine invertebrate animal counterparts. Marine Antioxidants, 19(1):285-294.
- Pazla, R., Yanti, G., Jamarun, N., Zain, M., Triani, H. D., Putri, E. M., & Srifani, A. (2024). Identification of phytase producing bacteria from acidifying Tithonia diversifolia: Potential for ruminant feed development. Saudi Journal of Biological Sciences, 31(7):104006-104016.
- Puspantari, W., Kusnandar, F., Nuryani Lioe, H., & Laily, N. (2020). The inhibition of fucoidan fraction from Sargassum polycystum and Turbinaria conoides to α-amylase and α-glucosidase. Jurnal Pengolahan Hasil Perikanan Indonesia, 23(1):122-136.
- Qiu, Y., Jiang, H., Dong, Y., Wang, Y., Hamouda, H., I., Balah, M., A., & Mao, X. (2023). Expression and biochemical characterization of a novel fucoidanase from Flavobacterium algicola with the principal product of fucoidan-derived disaccharide. Foods, 11(7):10-25.
- Rasin, A. B., Silchenko, A. S., Kusaykin, M. I., Malyarenko, O. S., Zueva, A. O., Kalinovsky, A. I., Airong, J., Surits, V. V., & Ermakova, S. P. (2020). Enzymatic transformation and anti-tumor activity of Sargassum horneri fucoidan. Carbohydrate Polymers, 246(10):1-17.
- Saetan, U., Nontasak, P., Palasin, K., Saelim, H., Wonglapsuwan, M., Mayakun, J., Pongparadon, S., & Chotigeat, W. (2021). Potential health benefits of fucoidan from the brown seaweeds Sargassum plagiophyllum and Sargassum polycystum. Journal of Applied Phycology, 33(5):3357-3364.
- Sahu, O., & Singh, N. (2019). Significance of bioadsorption process on textile industry wastewater. In the impact and prospects of green chemistry for textile technology. American: Woodhead Publishing. 18(11):367-416.
- Sawant, S. S., Salunke, B. K., & Kim, B. S. (2015). A rapid, sensitive, simple plate assay for detection of microbial alginate lyase activity. Enzyme and Microbial Technology, 77(9):8-13.
- Seiler, H., Schmidt, V., Wenning, M., & Scherer, S. (2012). Bacillus kochii sp. nov., isolated from foods and a pharmaceuticals manufacturing site. International Journal of Systematic and Evolutionary Microbiology, 62(5):1092-1097.
- Setyawan, A., Isnansetyo, A., Murwantoko., Indarjulianto, S., & Handayani, C. R. (2018). Comparative immune response of dietary fucoidan from three Indonesian brown algae in white shrimp Litopenaeus vannamei. AACL Bioflux, 11(6):1707-1723.
- Setyawan, A., Juliasih, N. L. G. R., Darmawan, M., Susanto, G. N., & Sarida, M. (2023). Screening, characterization, and identification of fucoidanase-producing bacteria from Sargassum polycystum. AACL Bioflux, 16(3):1357-1371.
- Silchenko, A. S., Rasin, A. B., Kusaykin, M. I., Malyarenko, O. S., Shevchenko, N. M., Zueva, A. O., Kalinovsky, A. I., Zvyagintseva, T. N., & Ermakova, S. P. (2018). Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydrate Polymers, 193(1):189-195.
- Sinurat, E., & Kusumawati, R. (2017). Optimization of crude fucoidan extraction methods from brown seaweed Sargassum binderi Sonder. Jurnal Kelautan Dan Perikanan, 12(2):125-134.
- Sivagnanavelmurugan, M., Thaddaeus, B. J., Palavesam, A., & Immanuel, G. (2014). Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish & Shellfish Immunology, 39(2):439-449.
- Srimongkol, P., Songserm, P., Kuptawach, K., Puthong, S., Sangtanoo, P., Thitiprasert, S., Thongchul, N., Phunpruch, S., & Karnchanatat, A. (2022). Sulfated polysaccharides derived from marine microalgae, Synechococcus sp. VDW, inhibit the human colon cancer cell line Caco-2 by promoting cell apoptosis via the JNK and p38 MAPK signaling pathway. Algal Research, 69(1):1-23.
- Subaryono. (2019). Enzymatic production of alginate oligosaccharide (OSA) from the seaweed Sargassum crassifolium and its immunomodulatory activity. Dissertation. Bogor, Indonesia: IPB University.
- Sun, Q. L., Li, Y., Ni, L. Q., Li, Y. X., Cui, Y. S., Jiang, S. L., Xie, E. Y., Du, J., Deng, F., & Dong, C. X. (2020). Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydrate Polymers, 229(1):1-10.
- Sun, X., Yan, C., Fu, Y., Ai, C., Bi, J., Lin, W., & Song, S. (2023). Orally administrated fucoidan and its low-molecular-weight derivatives are absorbed differentially to alleviate coagulation and thrombosis. International Journal of Biological Macromolecules, 255(1):128092-128103.
- Sung, C., Wang, H., Sun, K., Hsieh, C., Huang, R., Sun, G., & Tang, S. (2022). Fucoidan from Sargassum hemiphyllum inhibits the stemness of cancer stem cells and epithelial-mesenchymal transitions in bladder cancer cells. International Journal of Biological Macromolecules, 221(11):623-633.
- Suresh, V., Senthilkumar, N., Thangam, R., Rajkumar, M., Anbazhagan, C., Rengasamy, R., Gunasekaran, P., Kannan, S., & Palani, P. (2013). Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochemistry, 48(2):364-373.
- Tran, V. H. N., Nguyen, T. T., Meier, S., Holck, J., Cao, H. T. T., Van, T. T. T., Meyer, A. S., & Mikkelsen, M. D. (2022). The endo-α(1,3)-fucoidanase mef2 releases uniquely branched oligosaccharides from Saccharina latissima fucoidans. Marine drugs, 20(5):1-22.
- Tuller, J., De Santis, C., & Jerry, D. R. (2012). Dietary influence of fucoidan supplementation on growth of Lates calcarifer (Bloch). Aquaculture Research, 45(4):1-6.
- Tzevelekidis, P., Theodosiou, M., Papadopoulou, A., Sakellis, E., Boukos, N., Bikogiannakis, A. K., Kyriakou, G., Efthimiadou, E. K., & Mitsopoulou, C. A. (2024). Visible-light-activated antibacterial and antipollutant properties of biocompatible Cu-doped and Ag-decorated TiO2 nanoparticles. Heliyon, 10(17):1-22.
- Vijayaram, S., Sun, Y., Zuorro, A., Ghafarifarsani, H., Van Doan, H., & Hoseinifar, S. H. (2022). Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish & Shellfish Immunology, 130(11):294-308.
- Wang, Y., Niu, D., Que, F., Li, Y., & Chen, Q. (2021). Low molecular weight fucoidan prepared by fucoidanase degradation – A promising browning inhibitor. LWT, 148(8):1-8.
- Wang, L., Wang, L., Tian, J., Yan, C., Song, C., Xiao, S., & Song, S. (2024). Characterization of low-molecular-weight fucoidan prepared by photocatalysis degradation and its regulation effect on metabolomics of LPS-stimulated macrophages. Food Bioscience, 61(10):104619-104628.
- Wang, Y., Xing, M., Cao, Q., Ji, A., Liang, H., & Song, S. (2019). Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Marine Drugs, 17(3):1-18.
- Yuliani, T. A., Anggoro, S., & Solichin, A. (2018). The influence of difference salinity on osmotic response, ionic regulation and growth of sidat (Anguilla sp.) elver phase during acclimation and cultivation period. Management of Aquatic Resources Journal, 7(4):333-341.
- Zainuddin, M., Pringgenies, D., Radjasa, O. K., Haeruddin, H., Sabdaningsih, A., & Herawati, V. E. (2022). Optimization of pH and salinity of culture media on growth and activity of extracellular protease bacteria Bacillus firmus from the seagrass padang ecosystem Nusa Lembongan Bali. Journal of Tropical Marine Science, 5(2):140-148.
- Zárate-Chaves, C. A., Romero-Rodríguez, M. C., Niño-Arias, F. C., Robles-Camargo, J., Linares-Linares, M., Rodríguez-Bocanegra, M. X., & Gutiérrez-Rojas, I. (2013). Optimizing a culture medium for biomass and phenolic compounds production using Ganoderma lucidum. Brazilian Journal of Microbiology, 44(1):215-223.
- Zhu, Y., Liu, L., Sun, Z., Ji, Y., Wang, D., Mei, L., Shen, P., Li, Z., Tang, S., Zhang, H., Zhou, Q., & Deng, J. (2021). Fucoidan as a marine-origin prebiotic modulates the growth and antibacterial ability of Lactobacillus rhamnosus. International Journal of Biological Macromolecules, 180(6):599-607.
- Zueva, A., Silchenko, A., Rasin, A., Kusaykin, M., Usoltseva, R., Kalinovsky, A., Kurilenko, V., Zvyagintseva, T., Thinh, P., & Ermakova, S. (2020). Expression and biochemical characterization of two recombinant fucoidanases from the marine bacterium Wenyingzhuangia fucanilytica CZ1127T. International Journal of Biological Macromolecules, 164(12):3025-3037.
References
Abbas, M. F., Karim, D. K., Kareem, H. R., Kamil, M. M., Al-Musawi, M. H., Asker, M. H., Ghanami, M., Shahriari-Khalaji, M., Sattar, M., Mirhaj, M., Sharifianjazi, F., Tavamaishvili, K., Mohabbatkhah, M., Soheily, A., Noory, P., & Tavakoli, M. (2025). Fucoidan and its derivatives: From extraction to cutting-edge biomedical applications. Carbohydrate Polymers, 357(6):123468-123476.
Abdel-Latif, H. M. R., Dawood, M. A. O., Alagawany, M., Faggio, C., Nowosad, J., & Kucharczyk, D. (2022). Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish and Shellfish Immunology, 122(3):115-130.
Anisha, G. S., Padmakumari, S., Patel, A. K., Pandey, A., & Singhania, R. R. (2022). Fucoidan from marine macroalgae: Biological actions and applications in regenerative medicine, drug delivery systems and food industry. Journal Bioengineering, 9(9):1-28.
Arisandi, A., Wardani, M. K., Badami, K., & Aranida, G. D. (2017). The impact of salinity differences on the viability of Vibrio fluvialis bacteria. Jurnal Ilmiah Perikanan Dan Kelautan, 9(2):91-97.
Barzkar, N., Rungsardthong, V., Tamadoni Jahromi, S., Laraib, Q., Das, R., Babich, O., & Sukhikh, S. (2023). A recent update on fucoidonase: Source, isolation methods and its enzymatic activity. Frontiers in Marine Science, 10(5):1-13.
Chen, S., & Li, D. (2023). Systematic and critical review on the antiviral bioactivities of complex carbohydrates against human noroviruses. Journal of Functional Foods, 108(9):1-8.
Cholaraj, R., & Venkatachalam, R. (2024). Investigation of antioxidant and anticancer potential of fucoidan (in-vitro & in-silico) from brown seaweed Padina boergesenii. Algal Research, 79(5):103442-103450.
Dumorné K, Córdova D. C, Astorga-Eló, M, & Renganathan, P. (2017). Extremozymes: A potential source for industrial applications. Journal of Microbiology and Biotechnology, 27(4):649-659.
Elshazly, T. M., Salvatori, D., Elattar, H., Bourauel, C., & Keilig, L. (2023). Effect of trimming line design and edge extension of orthodontic aligners on force transmission: A 3D finite element study. Journal of the Mechanical Behavior of Biomedical Materials, 140(4):105741-105750.
Fahmid, S., Jabeen, R., Mehar, S., Sajjad, N., Behlil, F., Riaz, M., Jameel, N., Ishtiyaq, H., Mukhtar, F., Khan, N., & Ali, J. (2023). Biomimetic synthesis and use of silver nanoparticles, an innocuous stratagem to combat fungal diseases in plants. Journal of Molecular Liquids, 391(12):123217-123225.
Fernando, I. P. S., Dias, M. K. H. M., Madusanka, D. M. D., Han, E. J., Kim, M. J., Heo, S., Lee, K., Cheong, S. H., & Ahn, G. (2021). Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. International Journal of Biological Macromolecules, 168(1):620-630.
Furqonita, A., Aritonang, A. B., & Wibowo, M. A. (2021). Synthesis of Bi3+ DOPED TiO2 and photocatalysis activity test of E. coli antibacteria under visible irradiation. Indonesian Journal of Pure and Applied Chemistry, 4(2):69-80.
Hikariastri, P., Winarno, H., Kusmardi, K., Laksmitawati, D. R., & Abdillah, S. (2019). Anti-inflammatory activity of fucoidan crude extract from Sargassum crassifolium against LPS-induced RAW 264.7 cells. Jurnal Kefarmasian Indonesia, 9(2):97-105.
Hu, S., Wang, J., Wang, J., Li, S., Jiang, W., & Liu, Y. (2017). Renoprotective effect of fucoidan from Acaudina molpadioides in streptozotocin/high fat diet-induced type 2 diabetic mice. Journal of Functional Foods, 31(4):123-130.
Ikeda-Ohtsubo, W., López Nadal, A., Zaccaria, E., Iha, M., Kitazawa, H., Kleerebezem, M., & Brugman, S. (2020). Intestinal microbiota and immune modulation in zebrafish by fucoidan from Okinawa Mozuku (Cladosiphon okamuranus). Frontiers in Nutrition, 7(6):1-12.
Kim, C., Alrefaei, R., Bushlaibi, M., Ndegwa, E., Kaseloo, P., & Wynn, C. (2019). Influence of growth temperature on thermal tolerance of leading foodborne pathogens. Food Science & Nutrition, 7(12):4027-4036.
Kuddus, M. (2019). Introduction to food enzymes. In M. Kuddus (Ed.), Enzymes in food biotechnology. Production, applications, and future prospects. (pp. 1-18). Academic Press.
Kusaykin, M. I., Silchenko, A. S., Zakharenko, A. M., & Zvyagintseva, T. N. (2016). Fucoidanases. Glycobiology, 26(1):3-12.
Li, Q., Jiang, C., Tan, H., Zhao, X., Li, K., & Yin, H. (2021). Characterization of recombinant E. coli expressing a novel fucosidase from Bacillus cereus 2–8 belonging to GH95 family. Protein Expression and Purification, 186(12):105897-105904.
Liu, S., Wang, Q., Shao, Z., Liu, Q., He, Y., Ren, D., Yang, H., & Li, X. (2023). Purification and characterization of the enzyme fucoidanase from Cobetia amphilecti utilizing fucoidan from Undaria pinnatifida. Foods, 12(7):1-17.
Ma, Y., Zhang, L., Ma, X., Bai, K., Tian, Z., Wang, Z., Muratkhan, M., Wang, X., Lü, X., & Liu, M. (2024). Saccharide mapping as an extraordinary method on characterization and identification of plant and fungi polysaccharides: A review. International Journal of Biological Macromolecules, 275(08):133350-133367.
Malyarenko, O. S., Malyarenko, T. V., Usoltseva, R. V., Silchenko, A. S., Kicha, A. A., Ivanchina, N. V., & Ermakova, S. P. (2021). Fucoidan from brown algae Fucus evanescens potentiates the anti-proliferative efficacy of asterosaponins from starfish Asteropsis carinifera in 2D and 3D models of melanoma cells. International Journal of Biological Macromolecules, 185(8):31-39.
Manivasagan, P. & Oh, J. (2015). Production of a novel fucoidanase for the green synthesis of gold nanoparticles by Streptomyces sp. and its cytotoxic effect on hela cell. Marine Drugs. 13(11):6818-6837.
Murtiyaningsih, H. & Hazmi, M. (2017). Isolation and cellulase enzyme activities assays in cellulolytic bacteria origin from soil waste. Agritrop – Journal of Agricultural Science. 15(2):293-308.
Nagappan, H., Pee, P. P., Kee, S. H. Y., Ow, J. T., Yan, S. W., Chew, L. Y., & Kong, K. W. (2017). Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: Low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Research International, 27(1):950-958.
Naoe, T., Hasebe, A., Horiuchi, R., Makita, Y., Okazaki, Y., Yasuda., Matsuo K., Yoshida, Y., Tsuga, K., Abe, Y & Yokoyama, A. (2020). Development of tissue conditioner containing cetylpyridinium chloride montmorillonite as new antimicrobial agent: Pilot study on antimicrobial activity and biocompatibility. Journal of Prosthodontic Research, 64(4):436-443.
Panjaitan, R. S., & Natalia, L. (2021). Extraction of sulfate polysaccharide from Sargassum polycystum using microwave-assisted extraction method and its toxicity test. Jurnal Pascapanen Dan Bioteknologi Kelautan Dan Perikanan, 16(1):23-32.
Pavão, M. S. G., & De Souza Cardoso, F. (2022). Seaweed fucoidans and their marine invertebrate animal counterparts. Marine Antioxidants, 19(1):285-294.
Pazla, R., Yanti, G., Jamarun, N., Zain, M., Triani, H. D., Putri, E. M., & Srifani, A. (2024). Identification of phytase producing bacteria from acidifying Tithonia diversifolia: Potential for ruminant feed development. Saudi Journal of Biological Sciences, 31(7):104006-104016.
Puspantari, W., Kusnandar, F., Nuryani Lioe, H., & Laily, N. (2020). The inhibition of fucoidan fraction from Sargassum polycystum and Turbinaria conoides to α-amylase and α-glucosidase. Jurnal Pengolahan Hasil Perikanan Indonesia, 23(1):122-136.
Qiu, Y., Jiang, H., Dong, Y., Wang, Y., Hamouda, H., I., Balah, M., A., & Mao, X. (2023). Expression and biochemical characterization of a novel fucoidanase from Flavobacterium algicola with the principal product of fucoidan-derived disaccharide. Foods, 11(7):10-25.
Rasin, A. B., Silchenko, A. S., Kusaykin, M. I., Malyarenko, O. S., Zueva, A. O., Kalinovsky, A. I., Airong, J., Surits, V. V., & Ermakova, S. P. (2020). Enzymatic transformation and anti-tumor activity of Sargassum horneri fucoidan. Carbohydrate Polymers, 246(10):1-17.
Saetan, U., Nontasak, P., Palasin, K., Saelim, H., Wonglapsuwan, M., Mayakun, J., Pongparadon, S., & Chotigeat, W. (2021). Potential health benefits of fucoidan from the brown seaweeds Sargassum plagiophyllum and Sargassum polycystum. Journal of Applied Phycology, 33(5):3357-3364.
Sahu, O., & Singh, N. (2019). Significance of bioadsorption process on textile industry wastewater. In the impact and prospects of green chemistry for textile technology. American: Woodhead Publishing. 18(11):367-416.
Sawant, S. S., Salunke, B. K., & Kim, B. S. (2015). A rapid, sensitive, simple plate assay for detection of microbial alginate lyase activity. Enzyme and Microbial Technology, 77(9):8-13.
Seiler, H., Schmidt, V., Wenning, M., & Scherer, S. (2012). Bacillus kochii sp. nov., isolated from foods and a pharmaceuticals manufacturing site. International Journal of Systematic and Evolutionary Microbiology, 62(5):1092-1097.
Setyawan, A., Isnansetyo, A., Murwantoko., Indarjulianto, S., & Handayani, C. R. (2018). Comparative immune response of dietary fucoidan from three Indonesian brown algae in white shrimp Litopenaeus vannamei. AACL Bioflux, 11(6):1707-1723.
Setyawan, A., Juliasih, N. L. G. R., Darmawan, M., Susanto, G. N., & Sarida, M. (2023). Screening, characterization, and identification of fucoidanase-producing bacteria from Sargassum polycystum. AACL Bioflux, 16(3):1357-1371.
Silchenko, A. S., Rasin, A. B., Kusaykin, M. I., Malyarenko, O. S., Shevchenko, N. M., Zueva, A. O., Kalinovsky, A. I., Zvyagintseva, T. N., & Ermakova, S. P. (2018). Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydrate Polymers, 193(1):189-195.
Sinurat, E., & Kusumawati, R. (2017). Optimization of crude fucoidan extraction methods from brown seaweed Sargassum binderi Sonder. Jurnal Kelautan Dan Perikanan, 12(2):125-134.
Sivagnanavelmurugan, M., Thaddaeus, B. J., Palavesam, A., & Immanuel, G. (2014). Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish & Shellfish Immunology, 39(2):439-449.
Srimongkol, P., Songserm, P., Kuptawach, K., Puthong, S., Sangtanoo, P., Thitiprasert, S., Thongchul, N., Phunpruch, S., & Karnchanatat, A. (2022). Sulfated polysaccharides derived from marine microalgae, Synechococcus sp. VDW, inhibit the human colon cancer cell line Caco-2 by promoting cell apoptosis via the JNK and p38 MAPK signaling pathway. Algal Research, 69(1):1-23.
Subaryono. (2019). Enzymatic production of alginate oligosaccharide (OSA) from the seaweed Sargassum crassifolium and its immunomodulatory activity. Dissertation. Bogor, Indonesia: IPB University.
Sun, Q. L., Li, Y., Ni, L. Q., Li, Y. X., Cui, Y. S., Jiang, S. L., Xie, E. Y., Du, J., Deng, F., & Dong, C. X. (2020). Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydrate Polymers, 229(1):1-10.
Sun, X., Yan, C., Fu, Y., Ai, C., Bi, J., Lin, W., & Song, S. (2023). Orally administrated fucoidan and its low-molecular-weight derivatives are absorbed differentially to alleviate coagulation and thrombosis. International Journal of Biological Macromolecules, 255(1):128092-128103.
Sung, C., Wang, H., Sun, K., Hsieh, C., Huang, R., Sun, G., & Tang, S. (2022). Fucoidan from Sargassum hemiphyllum inhibits the stemness of cancer stem cells and epithelial-mesenchymal transitions in bladder cancer cells. International Journal of Biological Macromolecules, 221(11):623-633.
Suresh, V., Senthilkumar, N., Thangam, R., Rajkumar, M., Anbazhagan, C., Rengasamy, R., Gunasekaran, P., Kannan, S., & Palani, P. (2013). Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochemistry, 48(2):364-373.
Tran, V. H. N., Nguyen, T. T., Meier, S., Holck, J., Cao, H. T. T., Van, T. T. T., Meyer, A. S., & Mikkelsen, M. D. (2022). The endo-α(1,3)-fucoidanase mef2 releases uniquely branched oligosaccharides from Saccharina latissima fucoidans. Marine drugs, 20(5):1-22.
Tuller, J., De Santis, C., & Jerry, D. R. (2012). Dietary influence of fucoidan supplementation on growth of Lates calcarifer (Bloch). Aquaculture Research, 45(4):1-6.
Tzevelekidis, P., Theodosiou, M., Papadopoulou, A., Sakellis, E., Boukos, N., Bikogiannakis, A. K., Kyriakou, G., Efthimiadou, E. K., & Mitsopoulou, C. A. (2024). Visible-light-activated antibacterial and antipollutant properties of biocompatible Cu-doped and Ag-decorated TiO2 nanoparticles. Heliyon, 10(17):1-22.
Vijayaram, S., Sun, Y., Zuorro, A., Ghafarifarsani, H., Van Doan, H., & Hoseinifar, S. H. (2022). Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish & Shellfish Immunology, 130(11):294-308.
Wang, Y., Niu, D., Que, F., Li, Y., & Chen, Q. (2021). Low molecular weight fucoidan prepared by fucoidanase degradation – A promising browning inhibitor. LWT, 148(8):1-8.
Wang, L., Wang, L., Tian, J., Yan, C., Song, C., Xiao, S., & Song, S. (2024). Characterization of low-molecular-weight fucoidan prepared by photocatalysis degradation and its regulation effect on metabolomics of LPS-stimulated macrophages. Food Bioscience, 61(10):104619-104628.
Wang, Y., Xing, M., Cao, Q., Ji, A., Liang, H., & Song, S. (2019). Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Marine Drugs, 17(3):1-18.
Yuliani, T. A., Anggoro, S., & Solichin, A. (2018). The influence of difference salinity on osmotic response, ionic regulation and growth of sidat (Anguilla sp.) elver phase during acclimation and cultivation period. Management of Aquatic Resources Journal, 7(4):333-341.
Zainuddin, M., Pringgenies, D., Radjasa, O. K., Haeruddin, H., Sabdaningsih, A., & Herawati, V. E. (2022). Optimization of pH and salinity of culture media on growth and activity of extracellular protease bacteria Bacillus firmus from the seagrass padang ecosystem Nusa Lembongan Bali. Journal of Tropical Marine Science, 5(2):140-148.
Zárate-Chaves, C. A., Romero-Rodríguez, M. C., Niño-Arias, F. C., Robles-Camargo, J., Linares-Linares, M., Rodríguez-Bocanegra, M. X., & Gutiérrez-Rojas, I. (2013). Optimizing a culture medium for biomass and phenolic compounds production using Ganoderma lucidum. Brazilian Journal of Microbiology, 44(1):215-223.
Zhu, Y., Liu, L., Sun, Z., Ji, Y., Wang, D., Mei, L., Shen, P., Li, Z., Tang, S., Zhang, H., Zhou, Q., & Deng, J. (2021). Fucoidan as a marine-origin prebiotic modulates the growth and antibacterial ability of Lactobacillus rhamnosus. International Journal of Biological Macromolecules, 180(6):599-607.
Zueva, A., Silchenko, A., Rasin, A., Kusaykin, M., Usoltseva, R., Kalinovsky, A., Kurilenko, V., Zvyagintseva, T., Thinh, P., & Ermakova, S. (2020). Expression and biochemical characterization of two recombinant fucoidanases from the marine bacterium Wenyingzhuangia fucanilytica CZ1127T. International Journal of Biological Macromolecules, 164(12):3025-3037.