
Date Log
Copyright (c) 2025 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
Evaluation of AMPEP as a Natural Biostimulant for Enhancing Biomass and Pigment Yield in Chlorella sorokiniana
Corresponding Author(s) : Jurmin H Sarri
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 17 No. 3 (2025): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Graphical Abstract
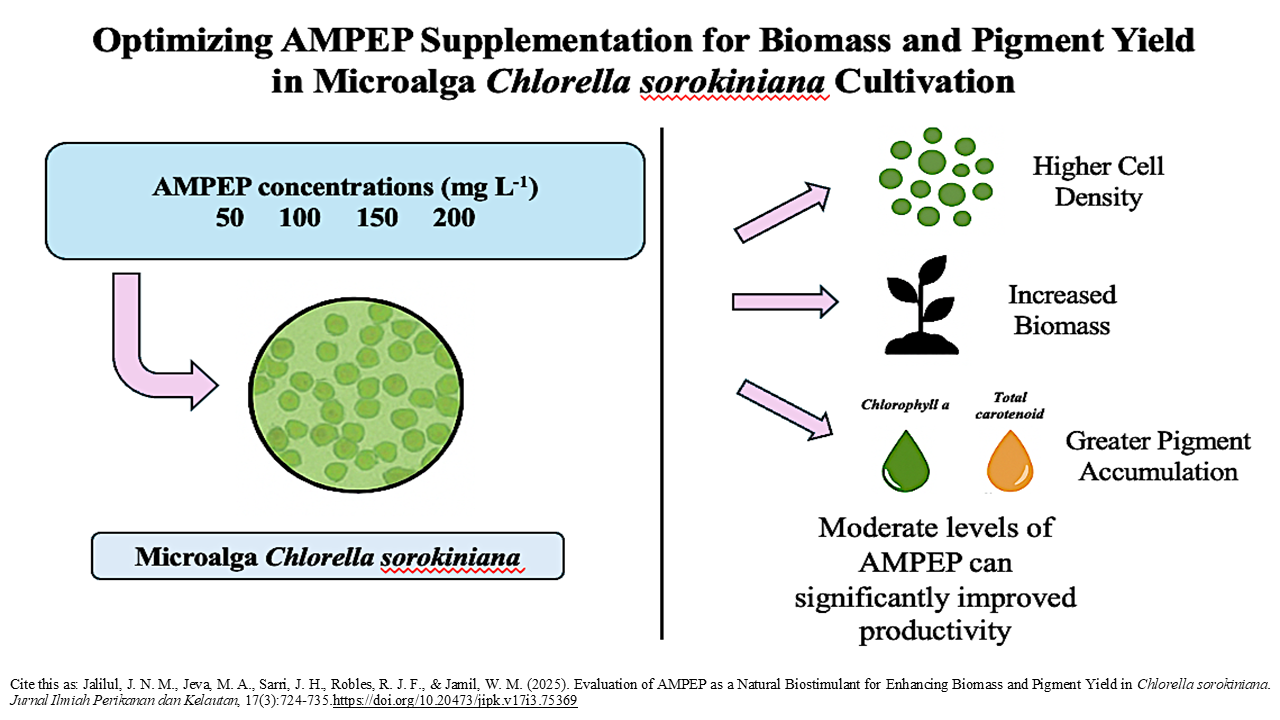
Highlight Research
- Chlorella sorokiniana achieved the highest cell density, growth rate, and biomass at 100 mg L⁻1 AMPEP.
- 100 mg L⁻¹ produced the largest cells, while higher concentrations (150–200 mg L⁻1) reduced cell density due to nutrient imbalances.
- 150 mg L⁻1 AMPEP maximized chlorophyll a and carotenoid accumulation, enhancing pigment production
- AMPEP demonstrated potential as a natural biostimulant to enhance microalgal productivity for biotechnological applications.
Abstract
Chlorella sorokiniana is a promising microalga valued for its production of pigments, lipids, and proteins with potential applications in biofuels, nutraceuticals, and pharmaceuticals. However, enhancing its growth and productivity remains a key challenge. Acadian Marine Plant Extract Powder (AMPEP), derived from the brown seaweed Ascophyllum nodosum, is known for its growth-promoting and stress-resistance properties in plants, but its effects on microalgae are not well understood. This study aimed to evaluate the effects of different concentrations of AMPEP (50, 100, 150, and 200 mg L⁻¹) on the growth, biomass, and pigment accumulation of C. sorokiniana. The experiment was conducted using a completely randomized design with five treatments (including a control) and three replicates per treatment. The results showed that 100 mg L⁻¹ AMPEP produced the highest cell density, with a 2.50-fold increase compared to the control, and the highest specific growth rate of 0.17 ± 0.03 day⁻¹. The largest cell size (19.51 ± 0.77 µm) was recorded at 200 mg L⁻¹, while biomass production peaked at 6.41 ± 0.49 g L⁻¹ with 50 mg L⁻¹. Maximum chlorophyll a and total carotenoid content were observed at 150 mg L⁻¹. Overall the 100 mg L⁻¹ AMPEP is the most balanced and optimal concentration overall for growth enhancement of C. sorokiniana, while other concentrations may be selected based on specific objectives like pigment or biomass production. These findings suggest that AMPEP, particularly at moderate concentrations, can significantly enhance the growth, biomass yield, and pigment content of C. sorokiniana. Further research is recommended to investigate the underlying mechanisms of AMPEP’s biostimulant effects and its potential application in large-scale algal cultivation systems.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Ahmad, A., Osman, S. M., Cha, T. S., & Loh, S. H. (2016). Phosphate-induced changes in fatty acid biosynthesis in Chlorella sp. KS-MA2 strain. Journal of Biotechnology, Computational Biology and Bionanotechnology, 97(4):295-304.
- Ak, I. (2012). Effect of an organic fertilizer on growth of blue-green alga Spirulina platensis. Aquaculture International, 20(4):413-422.
- Ali, O., Ramsubhag, A., & Jayaraman, J. (2021). Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants, 10(3):1-27.
- Anusree, M. K., Leela, K. M., Sreehari, M., Raj, S., Sreenikethanam, A., & Bajhaiya, A. K. (2023). Marine microalgae: An emerging source of pharmaceuticals and bioactive compounds. In S. N. Meena, V. Nandre, K. Kodam, & R. S. Meena (Eds.), New horizons in natural compound research. (pp. 251-265). Academic Press.
- Barsanti, L., & Gualtieri, P. (2022). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
- Briassoulis, D., Panagakis, P., Chionidis, M., Tzenos, D., Lalos, A., Tsinos, C., Berberidis, K., & Jacobsen, A. (2010). An experimental helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresource Technology, 101(17):6768-6777.
- Brito-Lopez, C., van der Wielen, N., Barbosa, M., & Karlova, R. (2025). Plant growth–promoting microbes and microalgae-based biostimulants: Sustainable strategy for agriculture and abiotic stress resilience. Philosophical Transactions of the Royal Society B, 380(1927):1-16.
- Chia, M. A., Lombardi, A. T., Melao, M. G. G., & Parrish, C. C. (2013). Lipid composition of Chlorella vulgaris (Trebouxiophyceae) as a function of different cadmium and phosphate concentrations. Aquatic Toxicology, 128(3):171-182.
- Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3):294-306.
- Craigie, J. S. (2011). Seaweed extract stimuli in plant science and agriculture. Journal of Applied Phycology, 23(3):371-393.
- Dahiya, S., Chowdhury, R., Tao, W., & Kumar, P. (2021). Biomass and lipid productivity by two algal strains of Chlorella sorokiniana grown in hydrolysate of water hyacinth. Energies, 14(5):1-21.
- De Clerck, O., Guiry, M. D., Leliaert, F., Samyn, Y., & Verbruggen, H. (2013). Algal taxonomy: A road to nowhere? Journal of Phycology, 49(2):215-225.
- Dragone, G., Fernandes, B. D., Abreu, A. P., Vicente, A. A., & Teixeira, J. A. (2011). Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Applied Energy, 88(10):3331-3335.
- Durmaz, Y., & Erbil, G. Ç. (2020). Comparison of industrial-scale tubular photobioreactor to FRP (fiberglass reinforced plastic) panel photobioreactor on outdoor culture of Nannochloropsis oculata in the marine hatchery. Ege Journal of Fisheries and Aquatic Sciences (EgeJFAS)/Su Ürünleri Dergisi, 37(3):303-308.
- El Boukhari, M. E. M., Barakate, M., Bouhia, Y., & Lyamlouli, K. (2020). Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants, 9(3):1-23.
- Erbil, G. C., & Durmaz, Y. (2020). Effects of myo-inositol concentration on growth and pigments of Nannochloropsis oculata culture. Ege Journal of Fisheries and Aquatic Sciences, 37(2):195-199.
- Erbil G. Ç., Durmaz Y., & Elp M. (2021) Indoor growth performance of Chlorella sp. production at tubular photobioreactor. Menba Journal of Fisheries Faculty, 7(2):90-95.
- Feng, P., Deng, Z., Fan, L., & Hu, Z. (2012). Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. Journal of Bioscience and Bioengineering, 114(4):405-410.
- Gaurav, K., Neeti, K., & Singh, R. (2024). Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technologies and Sustainability, 2(1):1-19.
- Gómez-Loredo, A., Benavides, J., & Rito-Palomares, M. (2016). Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. Journal of Applied Phycology, 28(3):849-860.
- Hadj-Romdhane, F., Zheng, X., Jaouen, P., Pruvost, J., Grizeau, D., Croué, J. P., & Bourseau, P. (2013). The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresource Technology, 132(6):285-292.
- Han, X., Zeng, H., Bartocci, P., Fantozzi, F., & Yan, Y. (2018). Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation, 4(2):1-15.
- Jaiswal, K. K., Banerjee, I., Singh, D., Sajwan, P., & Chhetri, V. (2020). Ecological stress stimulus to improve microalgae biofuel generation: A review. Octa Journal of Bioscience, 8(1):48-54.
- Khan, M. I., Shin, J. H., & Kim, J. D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories, 17(36):1-21.
- Khan, W., Rayirath, U. P., Subramanian, S., Jithesh, M. N., Rayorath, P., Hodges, D. M., Critchley, A. T., Craigie, J. S., Norrie, J., & Prithiviraj, B. (2009). Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation, 28(4):386-399.
- Kumara, N. T. R. N., Lim, A., Lim, C. M., Petra, M. I., & Ekanayake, P. (2017). Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renewable and Sustainable Energy Reviews, 78(13):301-317.
- Liu, C., Hu, B., Cheng, Y., Guo, Y., Yao, W., & Qian, H. (2021). Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresource Technology, 337(20):1-13.
- Macıas-Sánchez, M. D., Mantell, C., Rodrıguez, M., De La Ossa, E. M., Lubián, L. M., & Montero, O. (2005). Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. Journal of Food Engineering, 66(2):245-251.
- Manning, S. R., & Gol, R. D. (2021). Developments in algal processing. University of Texas at Austin, TX (United States).
- Miranda, A. M., Hernandez-Tenorio, F., Villalta, F., Vargas, G. J., & Sáez, A. A. (2024). Advances in the development of biofertilizers and biostimulants from microalgae. Biology, 13(3):1-19.
- Montoya-Vallejo, C., Duque, F. L. G., & Díaz, J. C. Q. (2023). Biomass and lipid production by the native green microalgae Chlorella sorokiniana in response to nutrients, light intensity, and carbon dioxide: Experimental and modeling approach. Frontiers in Bioengineering and Biotechnology, 11(1):1-16.
- Morais, M. G. D., Colla, L. M., & Costa, J. A. V. (2024). Microalgae superfoods. In J. A. V. Costa, B. G. Mitchell, & J. Benemann (Eds.), Microalgal bioengineering. (pp. 281-294). Cham: Springer International Publishing.
- Ogbonna, J. C., Nweze, N. O., & Ogbonna, C. N. (2021). Effects of light on cell growth, chlorophyll, and carotenoid contents of Chlorella sorokiniana and Ankistrodesmus falcatus in poultry dropping medium. Journal of Applied Biology & Biotechnology, 9(2):157-163.
- Ozioko, F. U., Chiejina, N. V., & Ogbonna, J. C. (2015). Effect of some phytohormones on growth characteristics of Chlorella sorokiniana IAM-C212 under photoautotrophic conditions. African Journal of Biotechnology, 14(30):2367-2376.
- Rouphael, Y., & Colla, G. (2020). Biostimulants in agriculture. Frontiers in plant science, 11(1):1-7.
- Saide, A., Martínez, K. A., Ianora, A., & Lauritano, C. (2021). Unlocking the health potential of microalgae as sustainable sources of bioactive compounds. International Journal of Molecular Sciences, 22(9):1-40.
- Saini, D. K., Chakdar, H., Pabbi, S., & Shukla, P. (2020). Enhancing production of microalgal biopigments through metabolic and genetic engineering. Critical Reviews in Food Science and Nutrition, 60(3):391-405.
- Sarri, J. H., & Elp, M. (2024). Optimization of iron, phosphate, and salinity in nutrient medium using response surface methodology for enhancing biochemical composition in Chlorella sp. culture. Algal Research, 84(8):1-14.
- Sarri, J., Erbil G. Ç. & Elp M., (2024b). Impact of acadian marine plant extract powder (AMPEP) concentration in nutrient medium on the growth and lipid accumulation of Chlorella sp. culture. Journal of Agricultural Sciences, 30(4):658-667.
- Sarri, J., Erbil G. Ç., Elp M., & Kadak A. E., (2024c). Acceptability of different concentrations of Chlorella sp. in Filipino Delicacy Puto as coloring agent. Journal of Agricultural Sciences, 34(1):62-73.
- Sarri, J. H., Ibno D. C. V., Hassan R. K., & Hairol M. D. (2024a). Investigation of the effect of AMPEP concentration in nutrient medium on the cell density, growth response, and pigment accumulation of Nannochloropsis sp. culture. AACL Bioflux, 17(6):2886-2898.
- Savage, E., Nagle, N., Laurens, L. M. L., & Knoshaug, E. P. (2020). Nitrogen derived from combined algal processing supports algae cultivation for biofuels. Algal Research, 50(6):1-8.
- Shuba, E. S., & Kifle, D. (2018). Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renewable and Sustainable Energy Reviews, 81(1):743-755.
- Sun, H., Wang, Y., He, Y., Liu, B., Mou, H., Chen, F., & Yang, S. (2023). Microalgae-derived pigments for the food industry. Marine Drugs, 21(2):1-27.
- Wang, S., Chen, Y., Ghonimy, A., Yu, T., Gao, Y., Wu, Z., Wang, Q., & Zhang, D. (2020). L-carnitine supplementation improved population growth, photosynthetic pigment synthesis and antioxidant activity of marine Chlorella sp. Aquaculture Reports, 17(2):1-7.
- Wang, B., Li, Y., Wu, N., & Lan, C. Q. (2008). CO2 bio-mitigation using microalgae. Applied Microbiology and Biotechnology, 79(5):707-718.
- Xia, D., Qiu, W., Wang, X., & Liu, J. (2021). Recent advancements and future perspectives of microalgae-derived pharmaceuticals. Marine Drugs, 19(12):1-23.
- Yaakob, M. A., Mohamed, R. M. S. R., Al-Gheethi, A., Gokare, R. A., & Ambati, R. R. (2021). Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells, 10(2):1-19.
- Zhang, L., Chen, S., Yang, Y., Xie, S., Luo, L., Lu, Y., & Luan, T. (2024). Chlorophyll a acts as a natural photosensitizer to drive nitrate reduction in nonphotosynthetic microorganisms. Science of the Total Environment, 945(40):1-11.
- Zhou, C., Le, J., Hua, D., He, T., & Mao, J. (2019). Imaging analysis of chlorophyll fluorescence induction for monitoring plant water and nitrogen treatments. Measurement, 136(6):478-486.
- Ziganshina, E. E., Bulynina, S. S., & Ziganshin, A. M. (2020). Comparison of the photoautotrophic growth regimens of Chlorella sorokiniana in a photobioreactor for enhanced biomass productivity. Biology, 9(7):1-13.
- Zou, N., & Richmond, A. (2000). Light-path length and population density in photoacclimation of Nannochloropsis sp. (Eustigmatophyceae). Journal of Applied Phycology, 12(3):349-354.
References
Ahmad, A., Osman, S. M., Cha, T. S., & Loh, S. H. (2016). Phosphate-induced changes in fatty acid biosynthesis in Chlorella sp. KS-MA2 strain. Journal of Biotechnology, Computational Biology and Bionanotechnology, 97(4):295-304.
Ak, I. (2012). Effect of an organic fertilizer on growth of blue-green alga Spirulina platensis. Aquaculture International, 20(4):413-422.
Ali, O., Ramsubhag, A., & Jayaraman, J. (2021). Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants, 10(3):1-27.
Anusree, M. K., Leela, K. M., Sreehari, M., Raj, S., Sreenikethanam, A., & Bajhaiya, A. K. (2023). Marine microalgae: An emerging source of pharmaceuticals and bioactive compounds. In S. N. Meena, V. Nandre, K. Kodam, & R. S. Meena (Eds.), New horizons in natural compound research. (pp. 251-265). Academic Press.
Barsanti, L., & Gualtieri, P. (2022). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Briassoulis, D., Panagakis, P., Chionidis, M., Tzenos, D., Lalos, A., Tsinos, C., Berberidis, K., & Jacobsen, A. (2010). An experimental helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresource Technology, 101(17):6768-6777.
Brito-Lopez, C., van der Wielen, N., Barbosa, M., & Karlova, R. (2025). Plant growth–promoting microbes and microalgae-based biostimulants: Sustainable strategy for agriculture and abiotic stress resilience. Philosophical Transactions of the Royal Society B, 380(1927):1-16.
Chia, M. A., Lombardi, A. T., Melao, M. G. G., & Parrish, C. C. (2013). Lipid composition of Chlorella vulgaris (Trebouxiophyceae) as a function of different cadmium and phosphate concentrations. Aquatic Toxicology, 128(3):171-182.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3):294-306.
Craigie, J. S. (2011). Seaweed extract stimuli in plant science and agriculture. Journal of Applied Phycology, 23(3):371-393.
Dahiya, S., Chowdhury, R., Tao, W., & Kumar, P. (2021). Biomass and lipid productivity by two algal strains of Chlorella sorokiniana grown in hydrolysate of water hyacinth. Energies, 14(5):1-21.
De Clerck, O., Guiry, M. D., Leliaert, F., Samyn, Y., & Verbruggen, H. (2013). Algal taxonomy: A road to nowhere? Journal of Phycology, 49(2):215-225.
Dragone, G., Fernandes, B. D., Abreu, A. P., Vicente, A. A., & Teixeira, J. A. (2011). Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Applied Energy, 88(10):3331-3335.
Durmaz, Y., & Erbil, G. Ç. (2020). Comparison of industrial-scale tubular photobioreactor to FRP (fiberglass reinforced plastic) panel photobioreactor on outdoor culture of Nannochloropsis oculata in the marine hatchery. Ege Journal of Fisheries and Aquatic Sciences (EgeJFAS)/Su Ürünleri Dergisi, 37(3):303-308.
El Boukhari, M. E. M., Barakate, M., Bouhia, Y., & Lyamlouli, K. (2020). Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants, 9(3):1-23.
Erbil, G. C., & Durmaz, Y. (2020). Effects of myo-inositol concentration on growth and pigments of Nannochloropsis oculata culture. Ege Journal of Fisheries and Aquatic Sciences, 37(2):195-199.
Erbil G. Ç., Durmaz Y., & Elp M. (2021) Indoor growth performance of Chlorella sp. production at tubular photobioreactor. Menba Journal of Fisheries Faculty, 7(2):90-95.
Feng, P., Deng, Z., Fan, L., & Hu, Z. (2012). Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. Journal of Bioscience and Bioengineering, 114(4):405-410.
Gaurav, K., Neeti, K., & Singh, R. (2024). Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technologies and Sustainability, 2(1):1-19.
Gómez-Loredo, A., Benavides, J., & Rito-Palomares, M. (2016). Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. Journal of Applied Phycology, 28(3):849-860.
Hadj-Romdhane, F., Zheng, X., Jaouen, P., Pruvost, J., Grizeau, D., Croué, J. P., & Bourseau, P. (2013). The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresource Technology, 132(6):285-292.
Han, X., Zeng, H., Bartocci, P., Fantozzi, F., & Yan, Y. (2018). Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation, 4(2):1-15.
Jaiswal, K. K., Banerjee, I., Singh, D., Sajwan, P., & Chhetri, V. (2020). Ecological stress stimulus to improve microalgae biofuel generation: A review. Octa Journal of Bioscience, 8(1):48-54.
Khan, M. I., Shin, J. H., & Kim, J. D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories, 17(36):1-21.
Khan, W., Rayirath, U. P., Subramanian, S., Jithesh, M. N., Rayorath, P., Hodges, D. M., Critchley, A. T., Craigie, J. S., Norrie, J., & Prithiviraj, B. (2009). Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation, 28(4):386-399.
Kumara, N. T. R. N., Lim, A., Lim, C. M., Petra, M. I., & Ekanayake, P. (2017). Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renewable and Sustainable Energy Reviews, 78(13):301-317.
Liu, C., Hu, B., Cheng, Y., Guo, Y., Yao, W., & Qian, H. (2021). Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresource Technology, 337(20):1-13.
Macıas-Sánchez, M. D., Mantell, C., Rodrıguez, M., De La Ossa, E. M., Lubián, L. M., & Montero, O. (2005). Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. Journal of Food Engineering, 66(2):245-251.
Manning, S. R., & Gol, R. D. (2021). Developments in algal processing. University of Texas at Austin, TX (United States).
Miranda, A. M., Hernandez-Tenorio, F., Villalta, F., Vargas, G. J., & Sáez, A. A. (2024). Advances in the development of biofertilizers and biostimulants from microalgae. Biology, 13(3):1-19.
Montoya-Vallejo, C., Duque, F. L. G., & Díaz, J. C. Q. (2023). Biomass and lipid production by the native green microalgae Chlorella sorokiniana in response to nutrients, light intensity, and carbon dioxide: Experimental and modeling approach. Frontiers in Bioengineering and Biotechnology, 11(1):1-16.
Morais, M. G. D., Colla, L. M., & Costa, J. A. V. (2024). Microalgae superfoods. In J. A. V. Costa, B. G. Mitchell, & J. Benemann (Eds.), Microalgal bioengineering. (pp. 281-294). Cham: Springer International Publishing.
Ogbonna, J. C., Nweze, N. O., & Ogbonna, C. N. (2021). Effects of light on cell growth, chlorophyll, and carotenoid contents of Chlorella sorokiniana and Ankistrodesmus falcatus in poultry dropping medium. Journal of Applied Biology & Biotechnology, 9(2):157-163.
Ozioko, F. U., Chiejina, N. V., & Ogbonna, J. C. (2015). Effect of some phytohormones on growth characteristics of Chlorella sorokiniana IAM-C212 under photoautotrophic conditions. African Journal of Biotechnology, 14(30):2367-2376.
Rouphael, Y., & Colla, G. (2020). Biostimulants in agriculture. Frontiers in plant science, 11(1):1-7.
Saide, A., Martínez, K. A., Ianora, A., & Lauritano, C. (2021). Unlocking the health potential of microalgae as sustainable sources of bioactive compounds. International Journal of Molecular Sciences, 22(9):1-40.
Saini, D. K., Chakdar, H., Pabbi, S., & Shukla, P. (2020). Enhancing production of microalgal biopigments through metabolic and genetic engineering. Critical Reviews in Food Science and Nutrition, 60(3):391-405.
Sarri, J. H., & Elp, M. (2024). Optimization of iron, phosphate, and salinity in nutrient medium using response surface methodology for enhancing biochemical composition in Chlorella sp. culture. Algal Research, 84(8):1-14.
Sarri, J., Erbil G. Ç. & Elp M., (2024b). Impact of acadian marine plant extract powder (AMPEP) concentration in nutrient medium on the growth and lipid accumulation of Chlorella sp. culture. Journal of Agricultural Sciences, 30(4):658-667.
Sarri, J., Erbil G. Ç., Elp M., & Kadak A. E., (2024c). Acceptability of different concentrations of Chlorella sp. in Filipino Delicacy Puto as coloring agent. Journal of Agricultural Sciences, 34(1):62-73.
Sarri, J. H., Ibno D. C. V., Hassan R. K., & Hairol M. D. (2024a). Investigation of the effect of AMPEP concentration in nutrient medium on the cell density, growth response, and pigment accumulation of Nannochloropsis sp. culture. AACL Bioflux, 17(6):2886-2898.
Savage, E., Nagle, N., Laurens, L. M. L., & Knoshaug, E. P. (2020). Nitrogen derived from combined algal processing supports algae cultivation for biofuels. Algal Research, 50(6):1-8.
Shuba, E. S., & Kifle, D. (2018). Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renewable and Sustainable Energy Reviews, 81(1):743-755.
Sun, H., Wang, Y., He, Y., Liu, B., Mou, H., Chen, F., & Yang, S. (2023). Microalgae-derived pigments for the food industry. Marine Drugs, 21(2):1-27.
Wang, S., Chen, Y., Ghonimy, A., Yu, T., Gao, Y., Wu, Z., Wang, Q., & Zhang, D. (2020). L-carnitine supplementation improved population growth, photosynthetic pigment synthesis and antioxidant activity of marine Chlorella sp. Aquaculture Reports, 17(2):1-7.
Wang, B., Li, Y., Wu, N., & Lan, C. Q. (2008). CO2 bio-mitigation using microalgae. Applied Microbiology and Biotechnology, 79(5):707-718.
Xia, D., Qiu, W., Wang, X., & Liu, J. (2021). Recent advancements and future perspectives of microalgae-derived pharmaceuticals. Marine Drugs, 19(12):1-23.
Yaakob, M. A., Mohamed, R. M. S. R., Al-Gheethi, A., Gokare, R. A., & Ambati, R. R. (2021). Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells, 10(2):1-19.
Zhang, L., Chen, S., Yang, Y., Xie, S., Luo, L., Lu, Y., & Luan, T. (2024). Chlorophyll a acts as a natural photosensitizer to drive nitrate reduction in nonphotosynthetic microorganisms. Science of the Total Environment, 945(40):1-11.
Zhou, C., Le, J., Hua, D., He, T., & Mao, J. (2019). Imaging analysis of chlorophyll fluorescence induction for monitoring plant water and nitrogen treatments. Measurement, 136(6):478-486.
Ziganshina, E. E., Bulynina, S. S., & Ziganshin, A. M. (2020). Comparison of the photoautotrophic growth regimens of Chlorella sorokiniana in a photobioreactor for enhanced biomass productivity. Biology, 9(7):1-13.
Zou, N., & Richmond, A. (2000). Light-path length and population density in photoacclimation of Nannochloropsis sp. (Eustigmatophyceae). Journal of Applied Phycology, 12(3):349-354.