Date Log
Copyright (c) 2023 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
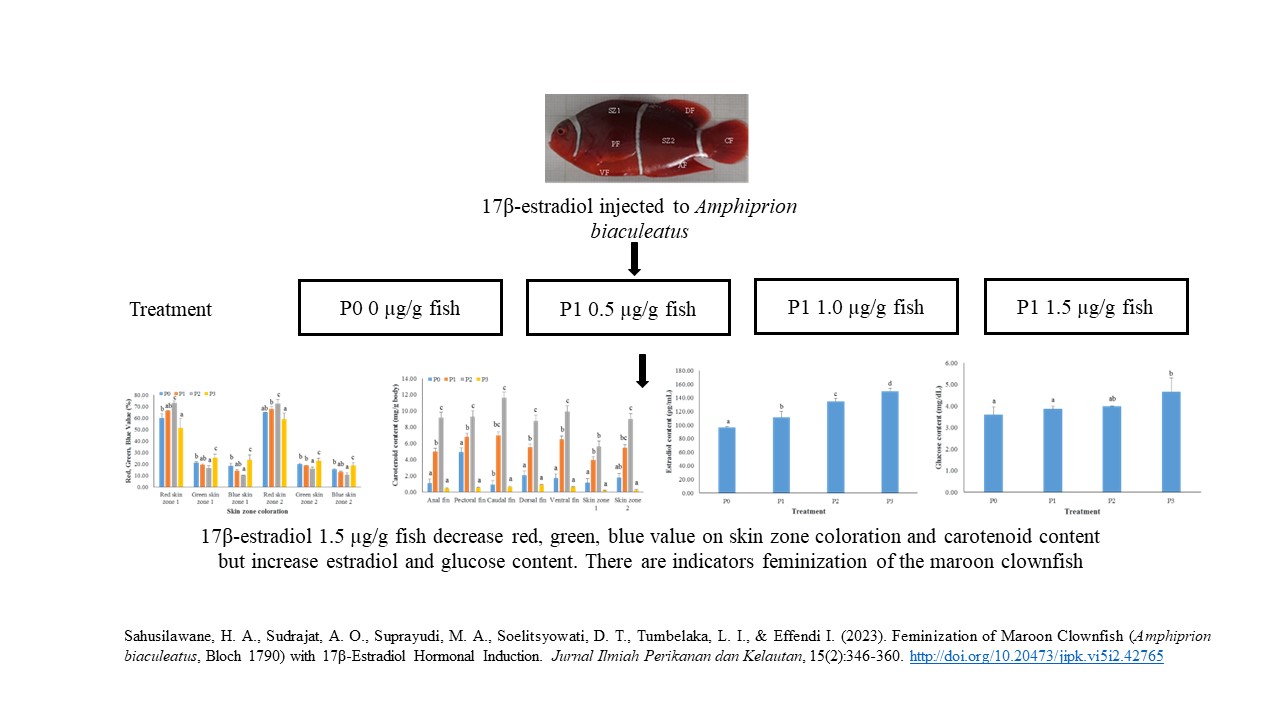
Feminization of Maroon Clownfish (Amphiprion biaculeatus, Bloch 1790) with 17β-Estradiol Hormonal Induction
Corresponding Author(s) : Agus Oman Sudrajat
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 15 No. 2 (2023): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Highlight Research
- The 17β-estradiol hormone induction reduces the percentage of R values in skin and fin color by as much as 50-60%.
- The 17β-estradiol hormone induction increases total length and body weight, estradiol content, GIS and HIS, and also glucose content.
- The 17β-estradiol hormone induction shows mature development of the gonadal profile from functional male to functional female.
Abstract
As maroon clownfish (Amphiprion biaculeatus) is a protandrous hermaphroditic fish, feminization process with 17β-estradiol hormone can be applied to accelerate the female broodstock candidate supply for further spawning effort. This study aimed to evaluate the feminization of A. biaculeatus with 17β-estradiol hormonal induction. This study used a completely randomized design with several hormone dosage, namely P0 (without 17β-estradiol hormone induction), P1 (0.5 μg 17β-estradiol/g body), P2 (1.0 μg 17β-estradiol/g body), and P3 (1.5 μg 17β-estradiol/g body). These treatments were applied with three replications. Five fish composed of α-fish, β-fish, and three γ-fish were reared in each aquarium for 90 days with a flowing water system. The α- and β-fish were then removed, while the γ-fish was injected with hormone. Otohime pellet feed was fed three times a day until apparent satiation. The results showed that the 17β-estradiol hormone could induce 100% of the feminization process of male A. biaculeatus. The dosage of P3 obtained the lowest value percentage of red, green, blue (RGB), but showing the highest total of length and body weight (6.67±0.42 cm and 6.40±0.78 g, respectively), estradiol content (149.73±4.24 Ïg/mL), GSI and HSI (0.38±0.07% and 3.59±0.49%), and glucose content (4.67±0.64 mg/dL), followed by more mature gonad profile than other treatments. This condition indicates that fish in P3 treatment has been reversed as functional female. The average survival rate for the treatment was as high as 60%. Therefore, the application of 17β-estradiol hormonal induction is effective for the feminization process in A. biaculeatus as a protandrous hermaphroditic fish.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Abol-Munafi, B. A., Norazmi-Lokman, N. H., Asma, N. A., Sarmiza, S., & Abduh, M. Y. (2011). Histological study on the gonad of the protandrous anemonefish (Amphiprion ocellaris). Journal of Animal and Veterinary Advances, 10(22):3031"’3036.
- Alonso-Alvarez, C., Pérez-Rodríguez, L., Mateo, R., Chastel, O., & Viñuela, J. (2008). The oxidation handicap hypothesis and the carotenoid allocation trade-off. Journal of Evolution Biology, 21(6):1789-1797.
- Biondo, M. V. (2018). Importation of marine ornamental fishes to Switzerland. Global Ecology and Conservation, 15:1-10.
- Casadevall, M., Delgado, E., Colleye, O., Monserrat, S. B., & Parmentier, E. (2009). Histological study of the sex-change in the skunk clownfish Amphiprion akallopisos. The Open Fish Science Journal, 2(1):55-58.
- Casas, L., Saborido-Rey, F., Ryu, T., Michell1, C., Ravasi, T., & Irigoien, X. (2016). Sex change in clownfish: molecular insights from transcriptome analysis. Scientific Reports, 6(35461):1-19.
- Casas, L., Parker, C. G., & Rhodes, J. S. (2022). Sex change from male to female: active feminization of the brain, behavior, and gonads in anemonefish. In V. Laudet and T. Ravasi (Eds), Evolution, development and ecology of anemonefishes. (pp. 117-128). Florida: CRC Press.
- Cavraro, F., Gheno, G., Ganzerla, R., Zucchetta, M., Franzoi, P., & Malavasi, S. (2017). Habitat constraints on carotenoid-based coloration in a small euryhaline teleost. Ecology and Evolution, 8(9):4422-4430.
- Falahatkar, B., Poursaeid, S., Meknatkhah, B., Khara, H., & Efatpana, I. (2013). Long-term effects of intraperitoneal injection of estradiol17b on the growth and physiology of juvenile stellate sturgeon Acipenser stellatus. Fish Physiology and Biochemistry, 40:365-373.
- Fitz, K. S., Montes Jr, H. R., Thompson, D. M., & Pinsky, M. L. (2022). Isolation-by-distance and isolation-by-oceanography in Maroon Anemonefish (Amphiprion biaculeatus). Evolutionary Applications, 16(2):379-392.
- Fitzgerald, L. M. (2020). What happened to nemo: Population dynamics of the orange clownfish, Amphiprion percula over an eight-year time gap on Kimbe Island, Papua New Guinea. Thesis. Thuwal: King Abdullah University of Science and Technology.
- Gemmell, N. J., Todd, E. V., Goikoetxea, A., Ortega-Recalde, O., & Hore, T. A. (2019). Natural sex change in fish. Current Topics in Development Biology, 134:71-177.
- Ghosh, S., Kumar, T. T. A., Gunasundari, S., & Balasubramanian. (2012). Reproductive biology of Amphiprion nigripes (Regan, 1908) at Lakshadweep archipelago: Implication for specific reef fish conservation in Asia. Scientific Reports, 1(1):2"’5.
- Glade, M. J., Smith, K., & Meguid, M. M. (2015). A glance at...nutritional antioxidants and testosterone secretion. Nutrition, 31(10):1295-1298.
- Geraghty, A. C., & Kaufer, D. (2015). Glucocorticoid Regulation of Reproduction. In W. E. Crusio, H. Dong, H. H. Radeke, N. Rezaei, O. Steinlein, J. Xiao (Eds.), Advances in experimental medicine and biology. (pp. 253-278). New York: Springer.
- Gopurappilly, R., Ogawa, S., & Parhar, I. S. (2013). Functional significanceof GnRH and kisspeptin, and their cognate receptors in teleost reproduction. Frontiers in Endrocrinology, 4(24):1-13.
- Haq, H. K., Santika, A., & Herawati, T. (2013). Pengaruh lama waktu perendaman induk dalam larutan madu terhadap pengalihan kelamin anak ikan Gapi (Poecilia reticulata). Jurnal Perikanan dan Kelautan, 4(3):117-125.
- Harlıoğlu, M. M., Yonar, M. E., Harlıoğlu, A. G., Yonar, S. A., Farhadi, A. (2018). Effects of 17β-estradiol injection on the reproductive efficiency of freshwater crayfish, Astacus leptodactylus (Eschscholtz, 1823). Journal of Applied Aquaculture.
- Ho, A. L. F. C., Zong, S., & Lin, J. (2014). Skin color retention after dietary carotenoid deprivation and dominance mediated skin coloration in clown anemonefish, Amphiprion ocellaris. AACL Bioflux, 7(2):103-115.
- Hoga, C. A., Almeida, F. L., & Reyes, F. G. R. (2018). A review on the use of hormones in fish farming: analytical methods to determine their residues. CYTA: Journal of Food, 16(1):679-691.
- Honeycutt, J. L. (2018). Environmental and endocrine regulators of stress effects in teleost fishes. Dissertation. North Carolina: North Carolina State University.
- Khoo, M. L., Das, S. K., & Ghaffar, M. A. (2018). Growth pattern, diet and reproductive biology of the clownfish Amphiprion ocellaris in waters of Pulau Tioman, Malaysia. The Egyptian Journal of Aquatic Research, 44(3):233-239.
- Kim, N. N., Jin, D. H., Lee, J., Kil, G. S., & Choi, C. Y. (2010). Upregulation of estrogen receptor subtypes and vitellogenin mRNA in cinnamon clownfish Amphiprion melanopus during the sex change process: Profiles on effects of 17β-estradiol. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 157(2):198-204.
- Klann, M., Mercader, M., Salis, P., Reynaud, M., Roux, N., Laudet, V., & Besseau, L. (2021). Anemonefishes. In A. Boutet., B. Schierwater, B. (Ed.), Handbook of marine model organisms in experimental biology established and emerging. (pp. 443-459). New York: CRC Press.
- Kobayashi, Y., Nakamura, M., Sunobe, T., Usami, T., Kobayashi, T., Manabe, H., Paul-Prasanth, B., Suzuki, N., & Nagahama, Y. (2009). Sex change in the gobiid fish is mediated through rapid switching of gonadotropin receptors from ovarian to testicular portion or vice versa. Endocrinology, 150(3):1503-1511.
- Kobayashi, Y., Nagahama, Y., Nakamura, M. (2013). Diversity and plasticity of sex determination and differentiation in fishes. Sexual Development, 7:115-125.
- Koski, J., Xie, H. & Olson, I. R. (2015). Understanding social hierarchies: The natural and psychological foundations of status perception. Social Neuroscience, 10(5)527-550.
- Kumar, P., Arasu, A. R. T., Kailasam, M., Sukumarran, K, Subburj, R., Tyagraj, G., & Natarajan, M. (2015). Gonadal development and steroid hormone profile of wild caught grey mullet (Mugil cephalus). Biological. Rhythm Research, 4:601-610.
- Kumar, P., Behera, P., Biswas, G., & Ghosha, T.K. (2022). Oocyte growth, gonadosomatic index, hepatosomatic index and levels of reproductive hormones in goldspot mullet Planiliza parsia (Hamilton, 1822) reared in captivity. Indian Journal of Fisheries, 69(1):84-96.
- Lee, W. K., & Yang, S. W. (2002). Relationship between ovarian development and serum level of gonadal steroid hormones, and induction of oocyte maturation and ovulation in the cultured female Korean spotted see bass Lateolabrax maculatus (Jeom-nong-eo). Aquaculture, 207(1-2):169-183.
- Li, M., Sun, L., & Wang, D. (2019). Roles of estrogens in fish sexual plasticity and sex differentiation. General and Comparative Endocrinology, 277:9-16.
- Liu H., Todd, E. V., Lokman, M. P., Lamm, M. S., Godwin, J. R., & Gemmell, N. J. (2017). Sexual plasticity: A fishy tale. Molecular Reproduction and Development, 84(2):171-194.
- Madhu, K., Madhu, R., & Retheesh. (2012). Broodstock development, breeding, embryonic development and larviculture of spine-cheek anemonefish, Premnas biaculeatus (Bloch, 1790). Indian Journal of Fish, 59(1):65-75.
- Madhu, R., Madhu, K., & Retheesh, T. (2013). Breeding and seed production of clown fishes under captivity. CMFRI, 203-208.
- Mitchell, J. S. (2003). Social correlates of reproductive success in false clown anemonefish; subordinate group members do not pay-to-stay. Evolutionary Ecology Research, 5:89-104.
- Nagahama, Y., Chakraborty, T., Paul-Prasanth, B., Ohta, K., & Nakamura, M. (2021). Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiological Reviews, 101(3):1237-1308.
- Nunes, C., Silva, A., Soares, E., & Ganias, K. (2011). The use of hepatic and somatic indices and histological information to characterize the reproductive dynamics of Atlantic sardine Sardina pilchardus from the Portuguese coast. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science, 3(1):127-144.
- Pereira, T. S. B., Boscolo, C. N. P., & Batlouni, S. R. (2020). Use of 17β-estradiol for Leporinus macrocephalus feminization. Boletim do Instituto de Pesca, 46(2):1-7.
- Pham, H. Q., & Nguyen, A. V. (2019). Seasonal changes in hepatosomatic index, gonadosomatic index and plasma estradiol"17β level in captively reared female rabbit fish (Siganus guttatus). Aquaculture Research, 50(8):2191-2199.
- Pham, H. Q., Nguyen, A. T., Kjí¸rsvik, E., Nguyen, M. D., & Arukwe, A. (2012). Seasonal reproductive cycle of Waigieu seaperch (Psammoperca waigiensis). Aquaculture Research, 43(6):815-830.
- Prasetio, A. B., & Kusrini, E. (2012). Ikan hias laut: tantangan budidaya dan peluang bisnis. Media Akuakultur, 7(2):84-87.
- Putra, I. G. D. K., Wardiyanto, & Tarsim. (2012). Hormon testosteron dan estradiol 17β dalam plasma darah induk betina ikan baung (Mystus nemurus). Jurnal Rekayasa dan Teknologi Budidaya Perairan, 1(1):71-78.
- Ramamoorthy, K., Bhuvaneswari, S., Sankar, G., & Sakkaravarthi, K. (2010). Proximate composition and carotenoid content of natural carotenoid sources and its colour enhancement on marine ornamental fish Amphiprion ocellaris (Cuveir 1880). World Journal of Fish and Marine Sciences, 2(6):545-550.
- Reading, B. J., Sullivan, C. V., & Schilling, J. (2017). Vitellogenesis in fishes. Reference Module in Life Sciences, 1-12.
- Roux, N., Salis, P., Lambert, A., Logeux, V., Soulat, O., Romans, P., Frédérich, B., Lecchini, D., & Laudet, V. (2019). Staging and normal table of post-embryonic development of the clownfish (Amphiprion ocellaris). Developmental Dynamic, 284(7):545-568.
- Salis, P., Lorin, T., Lewis, V., Rey, C., Marcionetti, A., Escande, M. L., Roux, N., Besseau, L., Salamin, N., Sémon, M., Parichy, D., Volff, J. N., & Laudet, V. (2019). Developmental and comparative transcriptomic identification of iridophore contribution to white barring in clownfish. Pigment Cell and Melanoma Research, 32(3):391-402.
- Sambroni, E., Rolland, A. D., Lareyre, J. J., & Le Gac, F. (2013). FSH and LH have common and distinct effects on gene expression in rainbow trout testis. Journal of Molecular Endocrinology, 50(1):1-18.
- Santo, A. P., Susilo, U., & Wijayanti, G. E. (2014). Perkembangan oosit induk Osteochilus hasselti c.v. yang diberi hormon estradiol-17β dan pakan dengan kadar protein berbeda. Scripta Biologica, 1(1):33-42.
- Tao, W., Yuan, J., Zhou, L., Sun, L., Sun, Y., Yang, S., Li, M., Zeng, S., Huang, B., & Wang, D. (2013). Characterization of gonadal transcriptomes from Nile tilapia (Oreochromis niloticus) reveals differentially expressed genes. PLoS One, 8:e63604.
- Thuong, N. P., Sung, Y. Y., Ambak, M. A., & Abol-Munafi, A. B. (2017). The hormone 17β-estradiol promotes feminization of juvenile protandrous hermaphrodite false clownfish (Amphiprion ocellaris). Marine and Freshwater Behavior and Physiology, 50(3):195-204.
- Tian, H., Li, Y., Wang, W., Wu, P., & Ru, S. (2012). Exposure to monocrotophos pesticide during sexual development causes the feminization/demasculinization of the reproductive traits and a reduction in the reproductive success of male guppies (Poecilia reticulata). Toxicology and Applied Pharmacology, 263(2):163-170.
- Vidal-López, J. M., Contreras-Sánchez, W. M., Hernández-Franyutti, A., Contreras-García, M. de J., & Uribe-Aranzábal, M. C. (2019). Functional feminization of the Mexican snook (Centropomus poeyi) using 17β-estradiol in the diet. Latin American Journal of Aquatic Research, 47(2):240-250.
- Vinkler, M., & Albrecht, T. (2010). Carotenoid maintenance handicap and the physiology of carotenoid-based signalisation of health. Naturwissenschaften, 97:19-28.
- Wedemeyer, G. A., & Yasutake, W. T. (1977). Clinical methods for the assessment stress on fish health. Technical Paper of The U.S. Fish and Wildlife Service. Washington, D.C.: Department of The Interior Fish and Wildlife Service.
- Zhang, Y., Zhang, S., Lu, H., Zhang, L., & Zhang, W. (2014). Genes encoding aromatases in teleosts: Evolution and expression regulation. General and Comparative Endocrinology, 205:151-158.
References
Abol-Munafi, B. A., Norazmi-Lokman, N. H., Asma, N. A., Sarmiza, S., & Abduh, M. Y. (2011). Histological study on the gonad of the protandrous anemonefish (Amphiprion ocellaris). Journal of Animal and Veterinary Advances, 10(22):3031"’3036.
Alonso-Alvarez, C., Pérez-Rodríguez, L., Mateo, R., Chastel, O., & Viñuela, J. (2008). The oxidation handicap hypothesis and the carotenoid allocation trade-off. Journal of Evolution Biology, 21(6):1789-1797.
Biondo, M. V. (2018). Importation of marine ornamental fishes to Switzerland. Global Ecology and Conservation, 15:1-10.
Casadevall, M., Delgado, E., Colleye, O., Monserrat, S. B., & Parmentier, E. (2009). Histological study of the sex-change in the skunk clownfish Amphiprion akallopisos. The Open Fish Science Journal, 2(1):55-58.
Casas, L., Saborido-Rey, F., Ryu, T., Michell1, C., Ravasi, T., & Irigoien, X. (2016). Sex change in clownfish: molecular insights from transcriptome analysis. Scientific Reports, 6(35461):1-19.
Casas, L., Parker, C. G., & Rhodes, J. S. (2022). Sex change from male to female: active feminization of the brain, behavior, and gonads in anemonefish. In V. Laudet and T. Ravasi (Eds), Evolution, development and ecology of anemonefishes. (pp. 117-128). Florida: CRC Press.
Cavraro, F., Gheno, G., Ganzerla, R., Zucchetta, M., Franzoi, P., & Malavasi, S. (2017). Habitat constraints on carotenoid-based coloration in a small euryhaline teleost. Ecology and Evolution, 8(9):4422-4430.
Falahatkar, B., Poursaeid, S., Meknatkhah, B., Khara, H., & Efatpana, I. (2013). Long-term effects of intraperitoneal injection of estradiol17b on the growth and physiology of juvenile stellate sturgeon Acipenser stellatus. Fish Physiology and Biochemistry, 40:365-373.
Fitz, K. S., Montes Jr, H. R., Thompson, D. M., & Pinsky, M. L. (2022). Isolation-by-distance and isolation-by-oceanography in Maroon Anemonefish (Amphiprion biaculeatus). Evolutionary Applications, 16(2):379-392.
Fitzgerald, L. M. (2020). What happened to nemo: Population dynamics of the orange clownfish, Amphiprion percula over an eight-year time gap on Kimbe Island, Papua New Guinea. Thesis. Thuwal: King Abdullah University of Science and Technology.
Gemmell, N. J., Todd, E. V., Goikoetxea, A., Ortega-Recalde, O., & Hore, T. A. (2019). Natural sex change in fish. Current Topics in Development Biology, 134:71-177.
Ghosh, S., Kumar, T. T. A., Gunasundari, S., & Balasubramanian. (2012). Reproductive biology of Amphiprion nigripes (Regan, 1908) at Lakshadweep archipelago: Implication for specific reef fish conservation in Asia. Scientific Reports, 1(1):2"’5.
Glade, M. J., Smith, K., & Meguid, M. M. (2015). A glance at...nutritional antioxidants and testosterone secretion. Nutrition, 31(10):1295-1298.
Geraghty, A. C., & Kaufer, D. (2015). Glucocorticoid Regulation of Reproduction. In W. E. Crusio, H. Dong, H. H. Radeke, N. Rezaei, O. Steinlein, J. Xiao (Eds.), Advances in experimental medicine and biology. (pp. 253-278). New York: Springer.
Gopurappilly, R., Ogawa, S., & Parhar, I. S. (2013). Functional significanceof GnRH and kisspeptin, and their cognate receptors in teleost reproduction. Frontiers in Endrocrinology, 4(24):1-13.
Haq, H. K., Santika, A., & Herawati, T. (2013). Pengaruh lama waktu perendaman induk dalam larutan madu terhadap pengalihan kelamin anak ikan Gapi (Poecilia reticulata). Jurnal Perikanan dan Kelautan, 4(3):117-125.
Harlıoğlu, M. M., Yonar, M. E., Harlıoğlu, A. G., Yonar, S. A., Farhadi, A. (2018). Effects of 17β-estradiol injection on the reproductive efficiency of freshwater crayfish, Astacus leptodactylus (Eschscholtz, 1823). Journal of Applied Aquaculture.
Ho, A. L. F. C., Zong, S., & Lin, J. (2014). Skin color retention after dietary carotenoid deprivation and dominance mediated skin coloration in clown anemonefish, Amphiprion ocellaris. AACL Bioflux, 7(2):103-115.
Hoga, C. A., Almeida, F. L., & Reyes, F. G. R. (2018). A review on the use of hormones in fish farming: analytical methods to determine their residues. CYTA: Journal of Food, 16(1):679-691.
Honeycutt, J. L. (2018). Environmental and endocrine regulators of stress effects in teleost fishes. Dissertation. North Carolina: North Carolina State University.
Khoo, M. L., Das, S. K., & Ghaffar, M. A. (2018). Growth pattern, diet and reproductive biology of the clownfish Amphiprion ocellaris in waters of Pulau Tioman, Malaysia. The Egyptian Journal of Aquatic Research, 44(3):233-239.
Kim, N. N., Jin, D. H., Lee, J., Kil, G. S., & Choi, C. Y. (2010). Upregulation of estrogen receptor subtypes and vitellogenin mRNA in cinnamon clownfish Amphiprion melanopus during the sex change process: Profiles on effects of 17β-estradiol. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 157(2):198-204.
Klann, M., Mercader, M., Salis, P., Reynaud, M., Roux, N., Laudet, V., & Besseau, L. (2021). Anemonefishes. In A. Boutet., B. Schierwater, B. (Ed.), Handbook of marine model organisms in experimental biology established and emerging. (pp. 443-459). New York: CRC Press.
Kobayashi, Y., Nakamura, M., Sunobe, T., Usami, T., Kobayashi, T., Manabe, H., Paul-Prasanth, B., Suzuki, N., & Nagahama, Y. (2009). Sex change in the gobiid fish is mediated through rapid switching of gonadotropin receptors from ovarian to testicular portion or vice versa. Endocrinology, 150(3):1503-1511.
Kobayashi, Y., Nagahama, Y., Nakamura, M. (2013). Diversity and plasticity of sex determination and differentiation in fishes. Sexual Development, 7:115-125.
Koski, J., Xie, H. & Olson, I. R. (2015). Understanding social hierarchies: The natural and psychological foundations of status perception. Social Neuroscience, 10(5)527-550.
Kumar, P., Arasu, A. R. T., Kailasam, M., Sukumarran, K, Subburj, R., Tyagraj, G., & Natarajan, M. (2015). Gonadal development and steroid hormone profile of wild caught grey mullet (Mugil cephalus). Biological. Rhythm Research, 4:601-610.
Kumar, P., Behera, P., Biswas, G., & Ghosha, T.K. (2022). Oocyte growth, gonadosomatic index, hepatosomatic index and levels of reproductive hormones in goldspot mullet Planiliza parsia (Hamilton, 1822) reared in captivity. Indian Journal of Fisheries, 69(1):84-96.
Lee, W. K., & Yang, S. W. (2002). Relationship between ovarian development and serum level of gonadal steroid hormones, and induction of oocyte maturation and ovulation in the cultured female Korean spotted see bass Lateolabrax maculatus (Jeom-nong-eo). Aquaculture, 207(1-2):169-183.
Li, M., Sun, L., & Wang, D. (2019). Roles of estrogens in fish sexual plasticity and sex differentiation. General and Comparative Endocrinology, 277:9-16.
Liu H., Todd, E. V., Lokman, M. P., Lamm, M. S., Godwin, J. R., & Gemmell, N. J. (2017). Sexual plasticity: A fishy tale. Molecular Reproduction and Development, 84(2):171-194.
Madhu, K., Madhu, R., & Retheesh. (2012). Broodstock development, breeding, embryonic development and larviculture of spine-cheek anemonefish, Premnas biaculeatus (Bloch, 1790). Indian Journal of Fish, 59(1):65-75.
Madhu, R., Madhu, K., & Retheesh, T. (2013). Breeding and seed production of clown fishes under captivity. CMFRI, 203-208.
Mitchell, J. S. (2003). Social correlates of reproductive success in false clown anemonefish; subordinate group members do not pay-to-stay. Evolutionary Ecology Research, 5:89-104.
Nagahama, Y., Chakraborty, T., Paul-Prasanth, B., Ohta, K., & Nakamura, M. (2021). Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiological Reviews, 101(3):1237-1308.
Nunes, C., Silva, A., Soares, E., & Ganias, K. (2011). The use of hepatic and somatic indices and histological information to characterize the reproductive dynamics of Atlantic sardine Sardina pilchardus from the Portuguese coast. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science, 3(1):127-144.
Pereira, T. S. B., Boscolo, C. N. P., & Batlouni, S. R. (2020). Use of 17β-estradiol for Leporinus macrocephalus feminization. Boletim do Instituto de Pesca, 46(2):1-7.
Pham, H. Q., & Nguyen, A. V. (2019). Seasonal changes in hepatosomatic index, gonadosomatic index and plasma estradiol"17β level in captively reared female rabbit fish (Siganus guttatus). Aquaculture Research, 50(8):2191-2199.
Pham, H. Q., Nguyen, A. T., Kjí¸rsvik, E., Nguyen, M. D., & Arukwe, A. (2012). Seasonal reproductive cycle of Waigieu seaperch (Psammoperca waigiensis). Aquaculture Research, 43(6):815-830.
Prasetio, A. B., & Kusrini, E. (2012). Ikan hias laut: tantangan budidaya dan peluang bisnis. Media Akuakultur, 7(2):84-87.
Putra, I. G. D. K., Wardiyanto, & Tarsim. (2012). Hormon testosteron dan estradiol 17β dalam plasma darah induk betina ikan baung (Mystus nemurus). Jurnal Rekayasa dan Teknologi Budidaya Perairan, 1(1):71-78.
Ramamoorthy, K., Bhuvaneswari, S., Sankar, G., & Sakkaravarthi, K. (2010). Proximate composition and carotenoid content of natural carotenoid sources and its colour enhancement on marine ornamental fish Amphiprion ocellaris (Cuveir 1880). World Journal of Fish and Marine Sciences, 2(6):545-550.
Reading, B. J., Sullivan, C. V., & Schilling, J. (2017). Vitellogenesis in fishes. Reference Module in Life Sciences, 1-12.
Roux, N., Salis, P., Lambert, A., Logeux, V., Soulat, O., Romans, P., Frédérich, B., Lecchini, D., & Laudet, V. (2019). Staging and normal table of post-embryonic development of the clownfish (Amphiprion ocellaris). Developmental Dynamic, 284(7):545-568.
Salis, P., Lorin, T., Lewis, V., Rey, C., Marcionetti, A., Escande, M. L., Roux, N., Besseau, L., Salamin, N., Sémon, M., Parichy, D., Volff, J. N., & Laudet, V. (2019). Developmental and comparative transcriptomic identification of iridophore contribution to white barring in clownfish. Pigment Cell and Melanoma Research, 32(3):391-402.
Sambroni, E., Rolland, A. D., Lareyre, J. J., & Le Gac, F. (2013). FSH and LH have common and distinct effects on gene expression in rainbow trout testis. Journal of Molecular Endocrinology, 50(1):1-18.
Santo, A. P., Susilo, U., & Wijayanti, G. E. (2014). Perkembangan oosit induk Osteochilus hasselti c.v. yang diberi hormon estradiol-17β dan pakan dengan kadar protein berbeda. Scripta Biologica, 1(1):33-42.
Tao, W., Yuan, J., Zhou, L., Sun, L., Sun, Y., Yang, S., Li, M., Zeng, S., Huang, B., & Wang, D. (2013). Characterization of gonadal transcriptomes from Nile tilapia (Oreochromis niloticus) reveals differentially expressed genes. PLoS One, 8:e63604.
Thuong, N. P., Sung, Y. Y., Ambak, M. A., & Abol-Munafi, A. B. (2017). The hormone 17β-estradiol promotes feminization of juvenile protandrous hermaphrodite false clownfish (Amphiprion ocellaris). Marine and Freshwater Behavior and Physiology, 50(3):195-204.
Tian, H., Li, Y., Wang, W., Wu, P., & Ru, S. (2012). Exposure to monocrotophos pesticide during sexual development causes the feminization/demasculinization of the reproductive traits and a reduction in the reproductive success of male guppies (Poecilia reticulata). Toxicology and Applied Pharmacology, 263(2):163-170.
Vidal-López, J. M., Contreras-Sánchez, W. M., Hernández-Franyutti, A., Contreras-García, M. de J., & Uribe-Aranzábal, M. C. (2019). Functional feminization of the Mexican snook (Centropomus poeyi) using 17β-estradiol in the diet. Latin American Journal of Aquatic Research, 47(2):240-250.
Vinkler, M., & Albrecht, T. (2010). Carotenoid maintenance handicap and the physiology of carotenoid-based signalisation of health. Naturwissenschaften, 97:19-28.
Wedemeyer, G. A., & Yasutake, W. T. (1977). Clinical methods for the assessment stress on fish health. Technical Paper of The U.S. Fish and Wildlife Service. Washington, D.C.: Department of The Interior Fish and Wildlife Service.
Zhang, Y., Zhang, S., Lu, H., Zhang, L., & Zhang, W. (2014). Genes encoding aromatases in teleosts: Evolution and expression regulation. General and Comparative Endocrinology, 205:151-158.