Date Log
Copyright (c) 2024 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
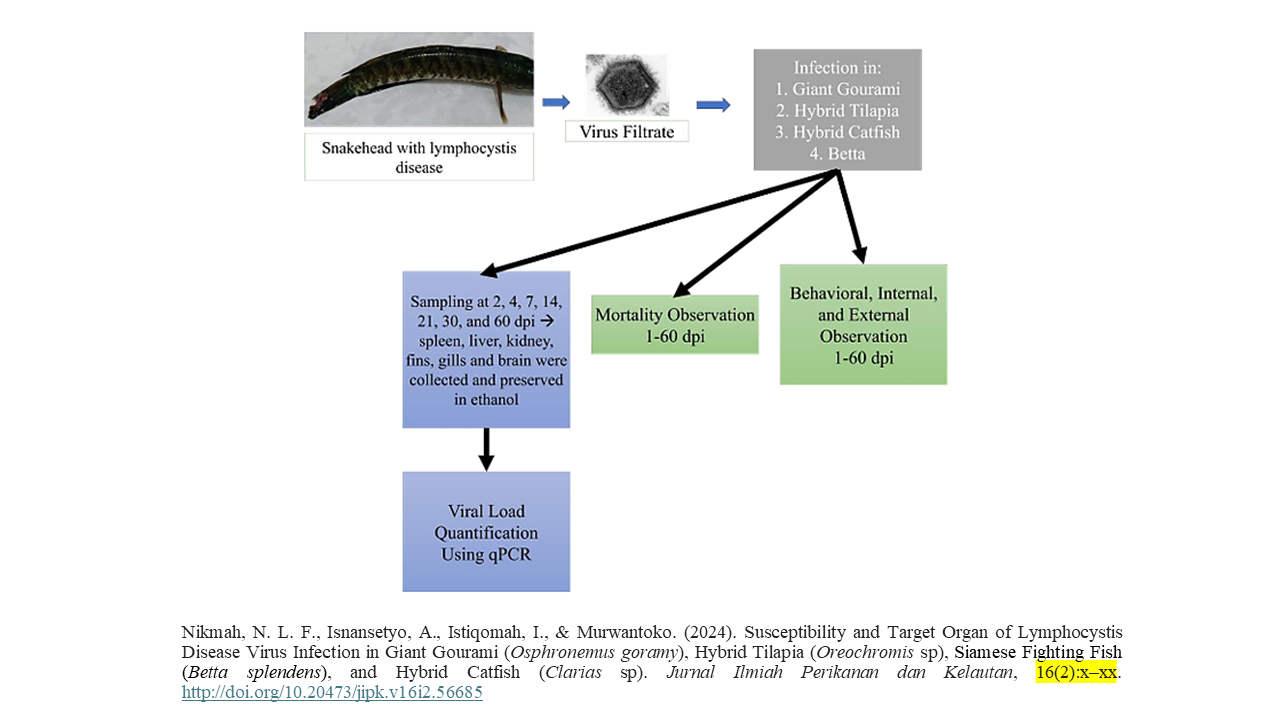
Susceptibility and Target Organ of Lymphocystis Disease Virus Infection in Giant Gourami (Osphronemus goramy), Hybrid Tilapia (Oreochromis sp.), Siamese Fighting Fish (Betta splendens), and Hybrid Catfish (Clarias sp.)
Corresponding Author(s) : Murwantoko Murwantoko
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 16 No. 2 (2024): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract

Highlight Research
1. Lymphocystis disease is reported to infect seawater and freshwater fishes
2. The four important freshwater fish species in Indonesia are evaluated on their susceptibility to Lymphocystis Disease Virus
3. Lymphocystis infection causes behavioural changes and mortality with different onset times after infection on the four fish species
4. LCDV load is varied in quantity among different organs.
Abstract
Lymphocystis disease has a broad host range and has been reported to enter Indonesia. However, information regarding its susceptibility and predilection organs in fish is lacking. This study examined the susceptibility of four important fish species in Indonesia, namely, giant gourami (Osphronemus goramy), hybrid tilapia (Oreochromis sp.), Siamese fighting fish (Bettasplendens), and hybrid catfish (Clarias sp.). The fish were infected with virus filtrate by intraperitoneal injection and immersion. The postinfection observation period was 60 days. Viral load was quantified by qPCR and expressed as major capsid protein (MCP) copy number/mg tissue. Mortality was observed in all fish species, with the highest recorded in hybrid catfish and the lowest in Siamese fighting fish. All the fish species showed changes in their clinical symptoms, such as anorexia and separation from schools. However, only giant gourami showed internal change seven days after injection (dpi), with white lesion detected in the liver. Viral load quantification showed that LCDV had different predilection organs in the four fish species. The highest viral load of giant gourami (1.7 x 104) was observed in the liver at 7 dpi, hybrid tilapia (7.5 x 103) was observed in the fins at 21 dpi, Siamese fighting fish (8.4 x 103) was observed in the fins at 14 dpi, and hybrid catfish (1.2 x 103) were observed in the fins and gills at 7 and 14 dpi. The findings indicated that giant gourami, hybrid tilapia, Siamese fighting fish, and hybrid catfish were susceptible to LCDV infection with different predilection organs.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Anasco, N. C., Koyama, J., & Uno, S. (2010). Pesticide residues in coastal waters affected by rice paddy effluents temporarily stored in a wastewater reservoir in Southern Japan. Archives of Environmental Contamination and Toxicology, 58(1):352-360.
- Aguayo-Quiroz, C. E., Leyva-Morales, J. B., Bastidas-Bastidas, P. J., Cruz-Acevedo, E., Zambrano-Soria, M., Granodes-Amores, J., Perea-Domingues, X. P., Soto-Alcala, J., Martinez-Alvarez, I. G., Salvatierra, V. D. C. Barreras-Serrano, A., & Gonzales-Marquez, L. C. (2020). Organochlorine pesticides in commercial fish species of the southeastern Gulf of California. Chemosphere, 257(1):1-32.
- Arias, A. H., & Botte, S. E. (Eds.). (2020). Coastal and deep ocean pollution. London: CRC Press.
- Burkow, I. C., & Kallenborn, R. (2000). Sources and transport of persistent pollutants to the Arctic. Toxicology Letters, 15(1):87-92.
- Charurvedi, S., & Dave, P. (2012). Microscopy in nanotechnology. Formatex, 946-952.
- Chung I. K., Sondak, C. F. A., & Beardall, J. (2017). The future of seaweed aquaculture in a rapidly changing world. European Journal of Phycology, 52(1):495-505.
- Ebenezer, V., & Ki, J. S. (2014). Quantification of toxic effects of organochlorine insecticide endosulfan on marine green algae, diatom, and dinoflagellate. Indian Journal of Geo-Marine Sciences, 43(3):393-3999.
- Gaston, C. P. (1994). Pesticide regulatory policies of selected countries in Asia. In: Technical Report No. 2. Bethesda, Maryland: Regional Agribusiness Project - RAP.
- Handini F R, Amin A dan Aguston. (2013). Effect Of Organochlorines (Endosulfan) Contaminated Medium on Content of Gelatin and Thallus Morphology Gracilaria verrucosa. Jurnal Ilmiah Perikanan dan Kelautan Vol. 5 No. 1, April 2013.
- Hu, L., Zhang, G., Zheng, B., Qi, Y. N., Lin, T., & Guo, Z. (2009). Occurrence and distribution of organochlorine pesticides (OCPs) in surface sediments of the Bohai Sea, China. Chemosphere, 77(5):663-672.
- Jayaraj, R., Megha, P., & Sreedev, P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary Toxicology, 9(3):90-100.
- Kepel, R. C., Lumingas, L. J. L., Tombokan, J. L., Desy, M. H., & Mantiri, D. M. H. (2021). Biomineral characterization and phytochemical profile of green algae Halimeda macroloba and Halimeda opuntia from coastal waters of Tanjung Merah, Bitung City, North Sulawesi, Indonesia. AACL Bioflux, 14(6):3217-3230.
- Neilson, A. H., Allard, A. S., Hynning, P. A., & Remberger, M. (1991). Distribution, fate and persistence of organochlorine compounds formed during the production of bleached pulp. Toxicological & Environmental Chemistry, 30(1):3-41.
- Nunes, L. M. (2022). Organochlorine compounds in beached plastics and marine organisms. Frontiers in Environmental Science, 9(784317):1-13.
- Perez, D. J., Iturburu, F. G., Calderon, G., Oyesqui, L. A., De Gerónimo, E., & Aparicio, V. C. (2021). Ecological risk assessment of current-use pesticides and biocides in soils, sediments, and surface water of a mixed land-use basin of the Pampas region, Argentina. Chemosphere, 263(1):1-14.
- Shahid, M., Manoharadas, S., Altaf, M., & Alrefaei, A.F. (2021). Organochlorine pesticides negatively influenced the cellular growth, morphostructure, cell viability, biofilm-formation, and phosphate-solubilization activities of Enterobacter cloacae strain EAM 35. ACS Omega, 6(8):5548-5559.
- Singkoh, M. F., Mantiri, D. M. H., Lumenta, C., & Manoppo, H. (2019). Biomineral characterization and antibacterial activity of marine algae Tricleocarpa fragilis from Kora-Kora coastal waters of Minahasa Regency, Indonesia. AACL Bioflux, 12(5):1814-1822.
- Sundhar, S., Jeyashakila, R., Jeyasekaran, G., Aanand, S., & Shalini, R. (2020). Assessment of organochlorine pesticides in different seaweed species of Thoothukudi coast, Tamil Nadu, South India. Indian Journal Fish, 68(2):148-153.
- Tang, C. Y., & Yang, Z. (2017). Transmission electron microscopy (TEM). In Membrane characterization (pp. 145-159). Elsevier.
- Tudi, M., Daniel, R. H., Wang, L., Lyu, J., Sadler, R., Connell, D., & Phung, D. T. (2021). Agriculture development, pesticide application and its impact on the environment. International Journal of Environmental Research and Public Health, 18(3):1-23.
- Vagi, M. C., Petsas, A. S., & Kostopoulou, M. N. (2021). Potential effects of persistent organic contaminants on marine biota: a review on recent research. Water, 13(18):1-35.
- Wolf, K. (1962). Experimental propagation of lymphocystis disease of fishes. Virology, 18(1):249-256.
- Xu, H. T., Piao, C. A., Jiang, Z. L., & Wang, W. X. (2000). Study on the causative agent of lymphocystis disease in cultured flounder Paralichthys olivaceus, in Mainland China. Chinese Journal of Virology, 16(3):223-226.
- Xu, L., Feng, J., & Huang, Y. (2014). Identification of lymphocystis disease virus from paradise fish Macropodus percularis (LCDV-PF). Archives of Virology, 159(9):2445-2449.
- Zhang, H., Sheng, X., Tang, X., Xing, J., Chi, H., & Zhan, W. (2023). Transcriptome analysis reveals molecular mechanisms of lymphocystis formation caused by lymphocystis disease virus infection in flounder (Paralichthys olivaceus). Frontiers in Immunology, 14(1):1-18.
- Zheng, F., Liu, H., Guo, X., & Wang, Bo. (2016). Isolation and identification of a new isolate of lymphocystis disease virus isolated from black rockfish (Sebastes schlegelii) in China, Aquaculture, 451(1):340-344.
References
Anasco, N. C., Koyama, J., & Uno, S. (2010). Pesticide residues in coastal waters affected by rice paddy effluents temporarily stored in a wastewater reservoir in Southern Japan. Archives of Environmental Contamination and Toxicology, 58(1):352-360.
Aguayo-Quiroz, C. E., Leyva-Morales, J. B., Bastidas-Bastidas, P. J., Cruz-Acevedo, E., Zambrano-Soria, M., Granodes-Amores, J., Perea-Domingues, X. P., Soto-Alcala, J., Martinez-Alvarez, I. G., Salvatierra, V. D. C. Barreras-Serrano, A., & Gonzales-Marquez, L. C. (2020). Organochlorine pesticides in commercial fish species of the southeastern Gulf of California. Chemosphere, 257(1):1-32.
Arias, A. H., & Botte, S. E. (Eds.). (2020). Coastal and deep ocean pollution. London: CRC Press.
Burkow, I. C., & Kallenborn, R. (2000). Sources and transport of persistent pollutants to the Arctic. Toxicology Letters, 15(1):87-92.
Charurvedi, S., & Dave, P. (2012). Microscopy in nanotechnology. Formatex, 946-952.
Chung I. K., Sondak, C. F. A., & Beardall, J. (2017). The future of seaweed aquaculture in a rapidly changing world. European Journal of Phycology, 52(1):495-505.
Ebenezer, V., & Ki, J. S. (2014). Quantification of toxic effects of organochlorine insecticide endosulfan on marine green algae, diatom, and dinoflagellate. Indian Journal of Geo-Marine Sciences, 43(3):393-3999.
Gaston, C. P. (1994). Pesticide regulatory policies of selected countries in Asia. In: Technical Report No. 2. Bethesda, Maryland: Regional Agribusiness Project - RAP.
Handini F R, Amin A dan Aguston. (2013). Effect Of Organochlorines (Endosulfan) Contaminated Medium on Content of Gelatin and Thallus Morphology Gracilaria verrucosa. Jurnal Ilmiah Perikanan dan Kelautan Vol. 5 No. 1, April 2013.
Hu, L., Zhang, G., Zheng, B., Qi, Y. N., Lin, T., & Guo, Z. (2009). Occurrence and distribution of organochlorine pesticides (OCPs) in surface sediments of the Bohai Sea, China. Chemosphere, 77(5):663-672.
Jayaraj, R., Megha, P., & Sreedev, P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary Toxicology, 9(3):90-100.
Kepel, R. C., Lumingas, L. J. L., Tombokan, J. L., Desy, M. H., & Mantiri, D. M. H. (2021). Biomineral characterization and phytochemical profile of green algae Halimeda macroloba and Halimeda opuntia from coastal waters of Tanjung Merah, Bitung City, North Sulawesi, Indonesia. AACL Bioflux, 14(6):3217-3230.
Neilson, A. H., Allard, A. S., Hynning, P. A., & Remberger, M. (1991). Distribution, fate and persistence of organochlorine compounds formed during the production of bleached pulp. Toxicological & Environmental Chemistry, 30(1):3-41.
Nunes, L. M. (2022). Organochlorine compounds in beached plastics and marine organisms. Frontiers in Environmental Science, 9(784317):1-13.
Perez, D. J., Iturburu, F. G., Calderon, G., Oyesqui, L. A., De Gerónimo, E., & Aparicio, V. C. (2021). Ecological risk assessment of current-use pesticides and biocides in soils, sediments, and surface water of a mixed land-use basin of the Pampas region, Argentina. Chemosphere, 263(1):1-14.
Shahid, M., Manoharadas, S., Altaf, M., & Alrefaei, A.F. (2021). Organochlorine pesticides negatively influenced the cellular growth, morphostructure, cell viability, biofilm-formation, and phosphate-solubilization activities of Enterobacter cloacae strain EAM 35. ACS Omega, 6(8):5548-5559.
Singkoh, M. F., Mantiri, D. M. H., Lumenta, C., & Manoppo, H. (2019). Biomineral characterization and antibacterial activity of marine algae Tricleocarpa fragilis from Kora-Kora coastal waters of Minahasa Regency, Indonesia. AACL Bioflux, 12(5):1814-1822.
Sundhar, S., Jeyashakila, R., Jeyasekaran, G., Aanand, S., & Shalini, R. (2020). Assessment of organochlorine pesticides in different seaweed species of Thoothukudi coast, Tamil Nadu, South India. Indian Journal Fish, 68(2):148-153.
Tang, C. Y., & Yang, Z. (2017). Transmission electron microscopy (TEM). In Membrane characterization (pp. 145-159). Elsevier.
Tudi, M., Daniel, R. H., Wang, L., Lyu, J., Sadler, R., Connell, D., & Phung, D. T. (2021). Agriculture development, pesticide application and its impact on the environment. International Journal of Environmental Research and Public Health, 18(3):1-23.
Vagi, M. C., Petsas, A. S., & Kostopoulou, M. N. (2021). Potential effects of persistent organic contaminants on marine biota: a review on recent research. Water, 13(18):1-35.
Wolf, K. (1962). Experimental propagation of lymphocystis disease of fishes. Virology, 18(1):249-256.
Xu, H. T., Piao, C. A., Jiang, Z. L., & Wang, W. X. (2000). Study on the causative agent of lymphocystis disease in cultured flounder Paralichthys olivaceus, in Mainland China. Chinese Journal of Virology, 16(3):223-226.
Xu, L., Feng, J., & Huang, Y. (2014). Identification of lymphocystis disease virus from paradise fish Macropodus percularis (LCDV-PF). Archives of Virology, 159(9):2445-2449.
Zhang, H., Sheng, X., Tang, X., Xing, J., Chi, H., & Zhan, W. (2023). Transcriptome analysis reveals molecular mechanisms of lymphocystis formation caused by lymphocystis disease virus infection in flounder (Paralichthys olivaceus). Frontiers in Immunology, 14(1):1-18.
Zheng, F., Liu, H., Guo, X., & Wang, Bo. (2016). Isolation and identification of a new isolate of lymphocystis disease virus isolated from black rockfish (Sebastes schlegelii) in China, Aquaculture, 451(1):340-344.