
Date Log
Copyright (c) 2025 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
A Filterable Agent Caused the Hemorrhagic Syndrome on Giant Gourami (Osphronemus goramy Lac.) at Yogyakarta
Corresponding Author(s) : Nur Lailatul Fitrotun Nikmah
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 17 No. 3 (2025): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Graphical Abstract
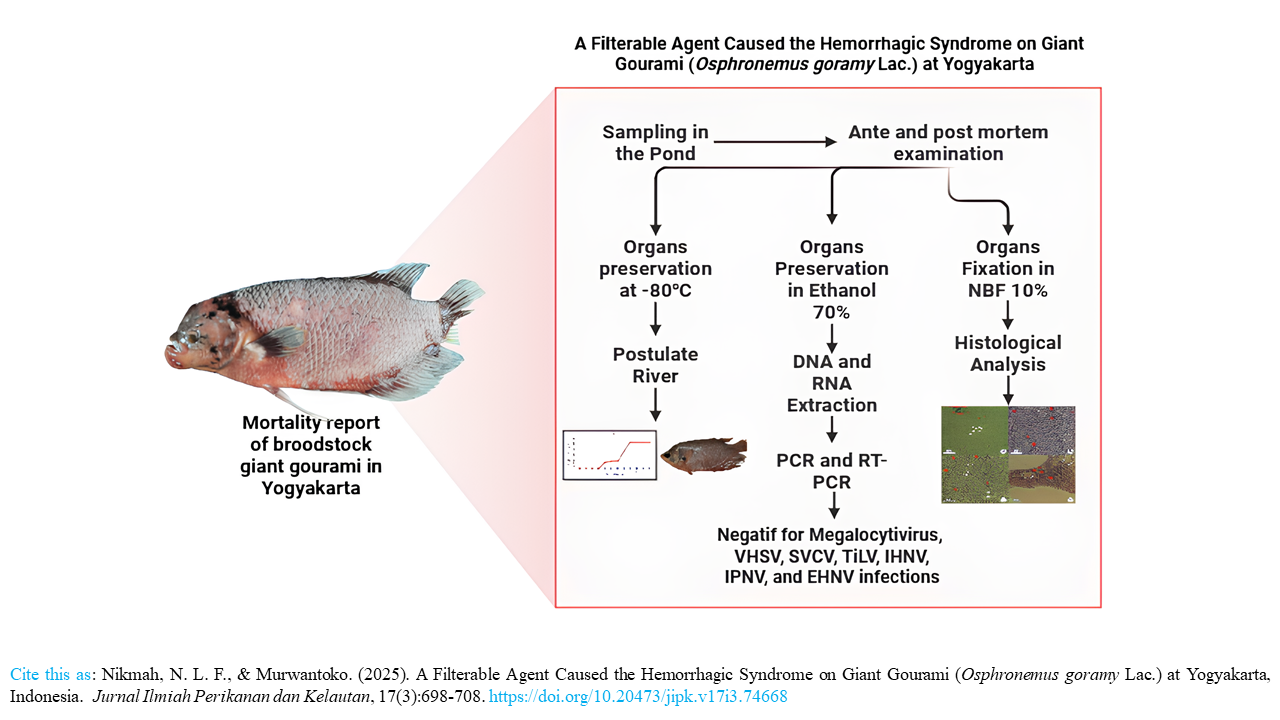
Highlight Research
- Yogyakarta reported a new disease outbreak that infected giant gourami (Osphronemus goramy Lac.) broodstock in several locations.
- Typical clinical symptoms of the outbreak include bleeding and visceral adhesions.
- The River’s postulate suggests that the cause of the outbreak is a filterable agent.
- The filterable agent has the potential to be a new species or strain of the virus.
Abstract
Giant gourami (Osphronemus goramy Lac.) is one of the important freshwater fish commodities in Indonesia. Disease infection is one of the constraints in the production of this fish. There have been reports of disease outbreaks that caused mortality in giant gourami in several locations in Yogyakarta, including Gamping and Moyudan Districts, Sleman Regency; and Wates District, Kulon Progo Regency. This study describes the disease based on observations of external and internal signs, along with the histopathology of several tissues. Postulate river is used to prove the causative disease of the filterable agents. Polymerase Chain Reaction (PCR) and Reverse Transcriptase PCR (RT-PCR) are applied to confirm the presence of the virus. Sick fishes show the hemorrhage over the entire body surface, rotted fins, exophthalmia, petechiae, pale liver, visceral adhesions, and enlarged kidneys. Histopathological analysis shows lipidosis in the liver; bleeding in the liver, kidneys, spleen, and brain; and multiple necrosis in the kidneys, spleen, and brain. Based on these signs, we designated the disease to be Hemorrhagic Syndrome. The River postulate test confirmed that virus was the causative agent of the disease, as infecting healthy fish with a bacteria-free filtrate homogenate from diseased fish organs resulted in the same clinical signs observed in a natural outbreak. PCR tests for Megalocytivirus and EHNV, along with RT-PCR tests for VHSV, SVCV, TiLV, IHNV, and IPNV, did not show any DNA bands, indicating that these viruses were not present. A filterable agent, potentially representing a new virus species or strain, causes hemorrhagic syndrome in giant gourami.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Agriandini, M., Sukenda, Widanarni, & Lusiastuti, A. M. (2021). Fate and tissue distribution of Mycobacterium fortuitum through immersion challenge as a model of natural infection in Osphronemus goramy. Aquaculture International, 29(6):1979-1989.
- Ahmadivand, S., Soltani, M., Shokrpoor, S., Ahmadzadeh, A., & Zargar, A. (2016). Isolation and identification of Viral Hemorrhagic Septicemia Virus (VHSV) from farmed rainbow trout (Oncorhynchus mykiss) in Iran. Iranian Journal of Fisheries Sciences, 15(3):1068-1078.
- Alturkistani, H. A., Tashkandi, F. M., & Mohammedsaleh, Z. M. (2016). Histological stains: A literature review and case study. Global Journal of Health Science, 8(3):72-79.
- Asgharian, H., Sahafi, H. H., Ardalan, A. A., Shekarriz, S., & Abdoli, A. (2011). Cytochrome C oxidase subunit 1 barcode data of fish of the Nayband National Park in the Persian Gulf and analysis using meta-data flag several cryptic species. Molecular Ecology Resources, 11(3):461-472.
- Ashraf, U., Lu, Y., Lin, L., Yuan, J., Wang, M., & Liu, X. (2016). Spring Viraemia of Carp Virus: Recent advances. The Journal of General Virology, 97(5):1037-1051.
- AVMA. (2020). AVMA guidelines for the euthanasia of animals: 2020 Edition. Schaumburg, IL: American Veterinary Medical Association.
- Azrita, A., Aryani, N., Mardiah, A., & Syandri, H. (2020). Growth, production and feed conversion performance of the gurami sago (Osphronemus goramy Lacepède, 1801) strain in different aquaculture systems. F1000Research, 9(161):1-16.
- Becker, J. A., Tweedie, A., Gilligan, D., Asmus, M., & Whittington, R. J. (2016). Susceptibility of Australian redfin perch (Perca fluviatilis) experimentally challenged with Epizootic Haematopoietic Necrosis Virus (EHNV). Journal of Aquatic Animal Health, 28(2):122-130.
- Bunnajirakul, S., Pavasutthipaisit, S., & Steinhagen, D. (2015). Pathological alterations due to motile Aeromonas infection in red swordtail fish (Xiphophorus helleri). Tierarztliche Praxis. Ausgabe K, Kleintiere/Heimtiere, 43(6):434-438.
- Chen, J., Tan, W., Wang, W., Hou, S., Chen, G., Xia, L., & Lu, Y. (2019). Identification of common antigens of three pathogenic Nocardia species and development of DNA vaccine against fish nocardiosis. Fish and Shellfish Immunology, 95(1):357-367.
- Deynez, G., Yılmaz, A., & Çelik, M. (2023). The role of anticoagulant, thrombolytic, and fibrinolytic activities in the formation and prevention of peritoneal adhesions. Trakya University Journal of Natural Sciences, 24(2):101-116.
- Dinh-Hung, N., Dong, H. T., Soontara, C., Rodkhum, C., Nimitkul, S., Srisapoome, P., Kayansamruaj, P., & Chatchaiphan, S. (2022). Co-infection of Candidatus Piscichlamydia Trichopodus (Order Chlamydiales) and Henneguya sp. (Myxosporea, Myxobolidae) in snakeskin gourami Trichopodus pectoralis (Regan 1910). Frontiers in Veterinary Science, 9(847977).
- Febrianti, R., Khasani, I., & Rosada, K. K. (2021). Assessing the susceptibility of the selected gourami (Osphronemus goramy) to Aeromonas hydrophila. Nusantara Bioscience, 13(1):111-120.
- Fitria, N., Handayani, N. A., Rahayu, W. P., Hidayat, T., & Rochima, E. (2021). Development of a co-agglutination method for detection of Aeromonas hydrophila as causative agent of motile aeromonas septicemia (MAS) disease in gourami (Osphronemus goramy). Iranian Journal of Fisheries Sciences, 20(1):123-135.
- García-Alegría, A. M., Anduro-Corona, I., Pérez-Martínez, C. J., Corella-Maduéno, M. A., Rascón-Durán, M. L., & Astiazaran-Garcia, H. (2020). Quantification of DNA through the nanodrop spectrophotometer: Methodological validation using standard reference material and sprague dawley rat and human DNA. International Journal of Analytical Chemistry, 2020(8896738).
- Gorgoglione, B., Bailey, C., & Ferguson, J. A. (2020). Proliferative kidney disease in Alaskan salmonids with evidence that pathogenic Myxozoans may be emerging north. International Journal for Parasitology, 50(10–11):797-807.
- Jaemwimol, P., Rawiwan, P., Tattiyapong, P., Saengnual, P., Kamlangdee, A., & Surachetpong, W. (2018). Susceptibility of important warm water fish species to Tilapia Lake Virus (TiLV) infection. Aquaculture, 497(1):462-468.
- Kurita, J., & Nakajima, K. (2012). Megalocytivirus. In A. M. Kibenge & M. G. Godoy (Eds.), Fish viruses and bacteria: Pathobiology and protection. (59-72). Dordrecht: Springer.
- Liakakos, T., Thomakos, N., Fine, P. M., Dervenis, C., & Young, R. L. (2001). Peritoneal adhesions: Etiology, pathophysiology, and clinical significance. Digestive Surgery, 18(4):260-273.
- Ludwig, M., Palha, N., Torhy, C., Briolat, V., Colucci-Guyon, E., Brémont, M., Herbomel, P., Boudinot, P., & Levraud, J. P. (2011). Whole-body analysis of a viral infection: vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PLoS Pathogens, 7(2):e1001269.
- Mishra, A. K., & Mohanty, B. (2009). Chronic exposure to sublethal hexavalent chromium affects organ histopathology and serum cortisol profile of a teleost, Channa punctatus (Bloch). The Science of the Total Environment, 407(18):5031-5038.
- Murwantoko, M., Bimantara, A., Roosmanto, R., & Kawaichi, M. (2016). Macrobrachium rosenbergii Nodavirus infection in a giant freshwater prawn hatchery in Indonesia. SpringerPlus, 5(1729).
- Murwantoko., Sari, D.W.K., Handayani, C.R., & Whittington, R.J. (2018). Genotype determination of megalocytivirus form Indonesian marine fishes. Biodiversitas, 19(15):1730-1736.
- Nagasawa, K. (2022). Do visceral adhesions affect the growth of sockeye salmon in the North Pacific Ocean and Bering Sea? Fish Pathology, 57(2):41-48.
- Nguyen, D. H., Dong, H. T., Taengphu, S., Soontara, C., Rodkhum, C., Senapin, S., & Chatchaiphan, S. (2023). Streptococcus suis is a lethal pathogen in snakeskin gourami, Trichopodus pectoralis. Aquaculture, 566(739173).
- Nguyen, D.-H., Dong, H. T., Phiwsaiya, K., Taengphu, S., Linh, N. V., Chatchaiphan, S., Rodkhum, C., Mai, H. N., Dhar, A. K., & Senapin, S. (2024). First report of natural infection with Infectious Spleen and Kidney Necrosis Virus (ISKNV) associated with disease outbreaks in two gourami species (Trichopodus spp.). SSRN.
- Noor El Deen, A. I. E., & Zaki, M. S. (2012). Eye affection syndrome in wild and cultured fish. Life Science Journal, 9(3):2568-2575.
- Patil, P. K., Geetha, R., Mishra, S. S., Abraham, T. J., Solanki, H. G., Sharma, S. R. K., Pradhan, P. K., Manna, S. K., Avunje, S., Abhinaya, D., Felix, K. T., Vinay, T. N., Paniprasad, K., Paria, A., Raja, S. A., Saraswathy, R., Sahoo, S. N., Rathod, R., Rameshkumar, P., Baitha, R., Thomas, S., Dev, A. K., Jayanthi, M., Swain, P., Sanil, N. K., & Jena, J. K. (2025). Unveiling the economic burden of diseases in aquatic animal food production in India. Frontiers in Sustainable Food Systems, 8(148009).
- Pierezan, F., Yun, S., Surachetpong, W., & Soto, E. (2020). Pathogenesis and immune response of Nile tilapia (Oreochromis niloticus) exposed to Tilapia Lake Virus by intragastric route. Journal of Fish Diseases, 43(12):1443-1454.
- Qin, P., Munang’andu, H. M., Xu, C., & Xie, J. (2023). Megalocytivirus and other members of the family iridoviridae in finfish: A review of the etiology, epidemiology, diagnosis, prevention, and control. Viruses, 15(1359):1-20.
- Rivers, T. M. (1937). Viruses and Koch’s postulates. Journal of Bacteriology, 33(1):1-12.
- Robles, F., Sandoval, C., Valdés, N., & Enríquez, R. (2022). Isolation of a new Infectious Pancreatic Necrosis Virus (IPNV) variant in Atlantic salmon (Salmo salar L.) that can cause high mortality even in genetically resistant fish. Frontiers in Genetics, 13(969252).
- Senthamarai, M. D., Rajan, M. R., & Bharathi, P. V. (2023). Current risks of microbial infections in fish and their prevention methods: A review. Aquaculture and Fisheries, 8(6):593-603.
- Slembrouck, J., Arifin, O. Z., Pouil, S., Subagja, J., Yani, A., Asependi, A., Kristanto, A. H., & Legendre, M. (2020). Seasonal variation of giant gourami (Osphronemus goramy) spawning activity and egg production in aquaculture ponds. Aquaculture, 527(735450).
- Souto, S., Lama, R., Mérour, E., Mehraz, M., Bernard, J., Lamoureux, A., Massaad, S., Frétaud, M., Rigaudeau, D., Millet, J. K., Langevin, C., & Biacchesi, S. (2024). In vivo multiscale analyses of Spring Viremia of Carp Virus (SVCV) infection: From model organism to target species. PLOS Pathogens, 20(8):e1012328.
- Subramaniam, K., Shariff, M., Omar, A. R., & Hair-Bejo, M. (2016). Megalocytivirus infection in fish: A review. Journal of Fish Diseases, 39(9):1195-1206.
- Sukenda, L., Gardenia, M. J., Zairin, M., Lusiastuti, A., & Alimudin. (2020). Identification of giant gourami iridovirus (GGIV): A new Infectious Spleen and Kidney Necrosis Virus (ISKNV) from natural outbreak in cultured Osphronemus goramy. Aquaculture International, 28(3):1069-1082.
- Swaminathan, T. R., Sundar Raj, N., Preena, P. G., Pradhan, P. K., Sood, N., Kumar, R. G., Sudhagar, A., & Sood, N. K. (2021). Infectious Spleen and Kidney Necrosis Virus-associated large-scale mortality in farmed giant gourami, Osphronemus goramy, in India. Journal of Fish Diseases, 44(12):1893-1900.
- Tattiyapong, P., Dachavichitlead, W., & Surachetpong, W. (2017). Experimental infection of Tilapia Lake Virus (TiLV) in Nile tilapia (Oreochromis niloticus) and red tilapia (Oreochromis spp.). Veterinary Microbiology, 207:170-177.
- Wang, H., Yang, H., Qiang, J., Kpundeh, M. D., Xu, P., & He, J. (2015). Evaluation and selection of appropriate reference genes for real-time quantitative PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus) during vaccination and infection. International Journal of Molecular Sciences, 16(5):9998-10015.
- Yong, C. Y., Ong, H. K., Tang, H. C., & Tan, W. S. (2019). Infectious Hematopoietic Necrosis Virus: Advances in diagnosis and vaccine development. PeerJ, 7:e7151.
References
Agriandini, M., Sukenda, Widanarni, & Lusiastuti, A. M. (2021). Fate and tissue distribution of Mycobacterium fortuitum through immersion challenge as a model of natural infection in Osphronemus goramy. Aquaculture International, 29(6):1979-1989.
Ahmadivand, S., Soltani, M., Shokrpoor, S., Ahmadzadeh, A., & Zargar, A. (2016). Isolation and identification of Viral Hemorrhagic Septicemia Virus (VHSV) from farmed rainbow trout (Oncorhynchus mykiss) in Iran. Iranian Journal of Fisheries Sciences, 15(3):1068-1078.
Alturkistani, H. A., Tashkandi, F. M., & Mohammedsaleh, Z. M. (2016). Histological stains: A literature review and case study. Global Journal of Health Science, 8(3):72-79.
Asgharian, H., Sahafi, H. H., Ardalan, A. A., Shekarriz, S., & Abdoli, A. (2011). Cytochrome C oxidase subunit 1 barcode data of fish of the Nayband National Park in the Persian Gulf and analysis using meta-data flag several cryptic species. Molecular Ecology Resources, 11(3):461-472.
Ashraf, U., Lu, Y., Lin, L., Yuan, J., Wang, M., & Liu, X. (2016). Spring Viraemia of Carp Virus: Recent advances. The Journal of General Virology, 97(5):1037-1051.
AVMA. (2020). AVMA guidelines for the euthanasia of animals: 2020 Edition. Schaumburg, IL: American Veterinary Medical Association.
Azrita, A., Aryani, N., Mardiah, A., & Syandri, H. (2020). Growth, production and feed conversion performance of the gurami sago (Osphronemus goramy Lacepède, 1801) strain in different aquaculture systems. F1000Research, 9(161):1-16.
Becker, J. A., Tweedie, A., Gilligan, D., Asmus, M., & Whittington, R. J. (2016). Susceptibility of Australian redfin perch (Perca fluviatilis) experimentally challenged with Epizootic Haematopoietic Necrosis Virus (EHNV). Journal of Aquatic Animal Health, 28(2):122-130.
Bunnajirakul, S., Pavasutthipaisit, S., & Steinhagen, D. (2015). Pathological alterations due to motile Aeromonas infection in red swordtail fish (Xiphophorus helleri). Tierarztliche Praxis. Ausgabe K, Kleintiere/Heimtiere, 43(6):434-438.
Chen, J., Tan, W., Wang, W., Hou, S., Chen, G., Xia, L., & Lu, Y. (2019). Identification of common antigens of three pathogenic Nocardia species and development of DNA vaccine against fish nocardiosis. Fish and Shellfish Immunology, 95(1):357-367.
Deynez, G., Yılmaz, A., & Çelik, M. (2023). The role of anticoagulant, thrombolytic, and fibrinolytic activities in the formation and prevention of peritoneal adhesions. Trakya University Journal of Natural Sciences, 24(2):101-116.
Dinh-Hung, N., Dong, H. T., Soontara, C., Rodkhum, C., Nimitkul, S., Srisapoome, P., Kayansamruaj, P., & Chatchaiphan, S. (2022). Co-infection of Candidatus Piscichlamydia Trichopodus (Order Chlamydiales) and Henneguya sp. (Myxosporea, Myxobolidae) in snakeskin gourami Trichopodus pectoralis (Regan 1910). Frontiers in Veterinary Science, 9(847977).
Febrianti, R., Khasani, I., & Rosada, K. K. (2021). Assessing the susceptibility of the selected gourami (Osphronemus goramy) to Aeromonas hydrophila. Nusantara Bioscience, 13(1):111-120.
Fitria, N., Handayani, N. A., Rahayu, W. P., Hidayat, T., & Rochima, E. (2021). Development of a co-agglutination method for detection of Aeromonas hydrophila as causative agent of motile aeromonas septicemia (MAS) disease in gourami (Osphronemus goramy). Iranian Journal of Fisheries Sciences, 20(1):123-135.
García-Alegría, A. M., Anduro-Corona, I., Pérez-Martínez, C. J., Corella-Maduéno, M. A., Rascón-Durán, M. L., & Astiazaran-Garcia, H. (2020). Quantification of DNA through the nanodrop spectrophotometer: Methodological validation using standard reference material and sprague dawley rat and human DNA. International Journal of Analytical Chemistry, 2020(8896738).
Gorgoglione, B., Bailey, C., & Ferguson, J. A. (2020). Proliferative kidney disease in Alaskan salmonids with evidence that pathogenic Myxozoans may be emerging north. International Journal for Parasitology, 50(10–11):797-807.
Jaemwimol, P., Rawiwan, P., Tattiyapong, P., Saengnual, P., Kamlangdee, A., & Surachetpong, W. (2018). Susceptibility of important warm water fish species to Tilapia Lake Virus (TiLV) infection. Aquaculture, 497(1):462-468.
Kurita, J., & Nakajima, K. (2012). Megalocytivirus. In A. M. Kibenge & M. G. Godoy (Eds.), Fish viruses and bacteria: Pathobiology and protection. (59-72). Dordrecht: Springer.
Liakakos, T., Thomakos, N., Fine, P. M., Dervenis, C., & Young, R. L. (2001). Peritoneal adhesions: Etiology, pathophysiology, and clinical significance. Digestive Surgery, 18(4):260-273.
Ludwig, M., Palha, N., Torhy, C., Briolat, V., Colucci-Guyon, E., Brémont, M., Herbomel, P., Boudinot, P., & Levraud, J. P. (2011). Whole-body analysis of a viral infection: vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PLoS Pathogens, 7(2):e1001269.
Mishra, A. K., & Mohanty, B. (2009). Chronic exposure to sublethal hexavalent chromium affects organ histopathology and serum cortisol profile of a teleost, Channa punctatus (Bloch). The Science of the Total Environment, 407(18):5031-5038.
Murwantoko, M., Bimantara, A., Roosmanto, R., & Kawaichi, M. (2016). Macrobrachium rosenbergii Nodavirus infection in a giant freshwater prawn hatchery in Indonesia. SpringerPlus, 5(1729).
Murwantoko., Sari, D.W.K., Handayani, C.R., & Whittington, R.J. (2018). Genotype determination of megalocytivirus form Indonesian marine fishes. Biodiversitas, 19(15):1730-1736.
Nagasawa, K. (2022). Do visceral adhesions affect the growth of sockeye salmon in the North Pacific Ocean and Bering Sea? Fish Pathology, 57(2):41-48.
Nguyen, D. H., Dong, H. T., Taengphu, S., Soontara, C., Rodkhum, C., Senapin, S., & Chatchaiphan, S. (2023). Streptococcus suis is a lethal pathogen in snakeskin gourami, Trichopodus pectoralis. Aquaculture, 566(739173).
Nguyen, D.-H., Dong, H. T., Phiwsaiya, K., Taengphu, S., Linh, N. V., Chatchaiphan, S., Rodkhum, C., Mai, H. N., Dhar, A. K., & Senapin, S. (2024). First report of natural infection with Infectious Spleen and Kidney Necrosis Virus (ISKNV) associated with disease outbreaks in two gourami species (Trichopodus spp.). SSRN.
Noor El Deen, A. I. E., & Zaki, M. S. (2012). Eye affection syndrome in wild and cultured fish. Life Science Journal, 9(3):2568-2575.
Patil, P. K., Geetha, R., Mishra, S. S., Abraham, T. J., Solanki, H. G., Sharma, S. R. K., Pradhan, P. K., Manna, S. K., Avunje, S., Abhinaya, D., Felix, K. T., Vinay, T. N., Paniprasad, K., Paria, A., Raja, S. A., Saraswathy, R., Sahoo, S. N., Rathod, R., Rameshkumar, P., Baitha, R., Thomas, S., Dev, A. K., Jayanthi, M., Swain, P., Sanil, N. K., & Jena, J. K. (2025). Unveiling the economic burden of diseases in aquatic animal food production in India. Frontiers in Sustainable Food Systems, 8(148009).
Pierezan, F., Yun, S., Surachetpong, W., & Soto, E. (2020). Pathogenesis and immune response of Nile tilapia (Oreochromis niloticus) exposed to Tilapia Lake Virus by intragastric route. Journal of Fish Diseases, 43(12):1443-1454.
Qin, P., Munang’andu, H. M., Xu, C., & Xie, J. (2023). Megalocytivirus and other members of the family iridoviridae in finfish: A review of the etiology, epidemiology, diagnosis, prevention, and control. Viruses, 15(1359):1-20.
Rivers, T. M. (1937). Viruses and Koch’s postulates. Journal of Bacteriology, 33(1):1-12.
Robles, F., Sandoval, C., Valdés, N., & Enríquez, R. (2022). Isolation of a new Infectious Pancreatic Necrosis Virus (IPNV) variant in Atlantic salmon (Salmo salar L.) that can cause high mortality even in genetically resistant fish. Frontiers in Genetics, 13(969252).
Senthamarai, M. D., Rajan, M. R., & Bharathi, P. V. (2023). Current risks of microbial infections in fish and their prevention methods: A review. Aquaculture and Fisheries, 8(6):593-603.
Slembrouck, J., Arifin, O. Z., Pouil, S., Subagja, J., Yani, A., Asependi, A., Kristanto, A. H., & Legendre, M. (2020). Seasonal variation of giant gourami (Osphronemus goramy) spawning activity and egg production in aquaculture ponds. Aquaculture, 527(735450).
Souto, S., Lama, R., Mérour, E., Mehraz, M., Bernard, J., Lamoureux, A., Massaad, S., Frétaud, M., Rigaudeau, D., Millet, J. K., Langevin, C., & Biacchesi, S. (2024). In vivo multiscale analyses of Spring Viremia of Carp Virus (SVCV) infection: From model organism to target species. PLOS Pathogens, 20(8):e1012328.
Subramaniam, K., Shariff, M., Omar, A. R., & Hair-Bejo, M. (2016). Megalocytivirus infection in fish: A review. Journal of Fish Diseases, 39(9):1195-1206.
Sukenda, L., Gardenia, M. J., Zairin, M., Lusiastuti, A., & Alimudin. (2020). Identification of giant gourami iridovirus (GGIV): A new Infectious Spleen and Kidney Necrosis Virus (ISKNV) from natural outbreak in cultured Osphronemus goramy. Aquaculture International, 28(3):1069-1082.
Swaminathan, T. R., Sundar Raj, N., Preena, P. G., Pradhan, P. K., Sood, N., Kumar, R. G., Sudhagar, A., & Sood, N. K. (2021). Infectious Spleen and Kidney Necrosis Virus-associated large-scale mortality in farmed giant gourami, Osphronemus goramy, in India. Journal of Fish Diseases, 44(12):1893-1900.
Tattiyapong, P., Dachavichitlead, W., & Surachetpong, W. (2017). Experimental infection of Tilapia Lake Virus (TiLV) in Nile tilapia (Oreochromis niloticus) and red tilapia (Oreochromis spp.). Veterinary Microbiology, 207:170-177.
Wang, H., Yang, H., Qiang, J., Kpundeh, M. D., Xu, P., & He, J. (2015). Evaluation and selection of appropriate reference genes for real-time quantitative PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus) during vaccination and infection. International Journal of Molecular Sciences, 16(5):9998-10015.
Yong, C. Y., Ong, H. K., Tang, H. C., & Tan, W. S. (2019). Infectious Hematopoietic Necrosis Virus: Advances in diagnosis and vaccine development. PeerJ, 7:e7151.