Impact of green tea (Camellia sinensis) leaf extract in skim milk-goose egg yolk semen extender on the quality of Sapudi ram spermatozoa stored at 5°C
Downloads
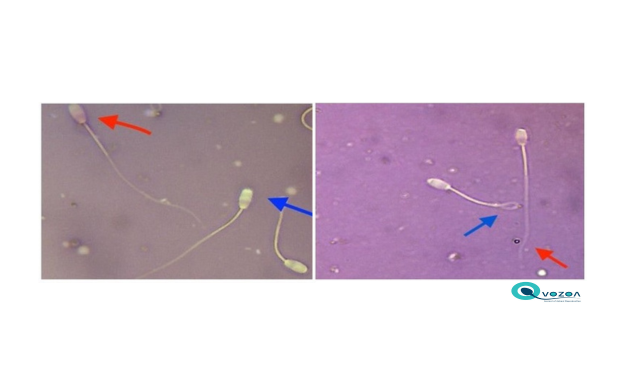
Livestock production requires Sapudi rams, a breed native to Indonesia, to meet meat demand and food security. In artificial high-quality frozen semen is needed to spread Sapudi rams. To maximize the survival of spermatozoa during cryopreservation, semen should be stored in an extender. Green tea leaf extract (GTLE) and skim milk-goat egg yolk (SM-GEY) may be a good cryoprotectants due to their antioxidant properties. This study aimed to determine the effect of adding GTLE to the SM-GEY extender on the quality of Sapudi ram spermatozoa stored at 5°C. The fresh semen sample was divided into four different GTLE treatment groups, which each contained a 0.1 mL semen sample and a 25-mL extender of SM-GEY. Group T0: no GTLE added to SM-GEY; Groups T1, T2, and T3: 0.1 mL semen diluted in 25 mL SM-GEY with 0.05, 0.10, and 0.15 mg GTLE. Extended semen was then stored at 5°C, and its quality was evaluated daily for five days. The variables observed included spermatozoa motility, viability, and membrane integrity. Data were analyzed using one-way analysis of variance followed by Duncan's test using Statistical Program and Service Solution version 23. The result of this study was that adding 0.05 mg GTLE to 25 mL of SM-GEY extender significantly maintained the spermatozoa motility, viability, and plasma membrane integrity of Sapudi ram spermatozoa for three days at 5°C (p <0.05). Therefore, it could be concluded that adding 0.05 mg of GTLE to the SM-GEY extender preserved Sapudi ram spermatozoa's motility, viability, and membrane integrity for three days at 5°C.
Alqawasmeh OA, Zhao M, Chan CP, Leung MB, Chow KC, Agarwal N, Mak JS, Wang CC, Pang CP, Li TC, Chu WK, Chan DY. 2021. Green tea extract as a cryoprotectant additive to preserve the motility and DNA integrity of human spermatozoa. Asian J Androl. 23: 150-6.
Ardiansyah R, Mirwandhono E, Hasnudi H. 2021. Analysis of development potential of sheep in Deli Serdang regency, North Sumatra province. J Peternakan Integratif 9: 71-8.
Ariyanto KB, Khotijah L, Astuti DA, Arifiantini RI, Menassol J-B. 2020. Semen quality of Garut rams feed by different protein sources and their implementation potential in small farms of West Java. J Agripet 20: 47-55.
Bernatoniene J, Kopustinskiene DM. 2018. The role of catechins in cellular responses to oxidative stress. Molecules 23: 965.
Bustani GS, Baiee FH. 2021. Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders. Vet World 14: 1220-33.
Chacko SM, Thambi PT, Kuttan R, Nishigaki I. 2010. Beneficial effects of green tea: a literature review. Chin Med. 5: 13.
Collodel G, Castellini C, Lee JC, Signorini C. 2020. Relevance of fatty acids to sperm maturation and quality. Oxid Med Cell Longev. 2020: 7038124.
Cseh S, Faigl V, Amiridis GS. 2012. Semen processing and artificial insemination in health management of small ruminants. Anim Reprod Sci. 130: 187-92.
Di Iorio M, Manchisi A, Rocco M, Chrenek P, Iaffaldano N. 2014. Comparison of different extenders on the preservability of rabbit semen stored at 5°C for 72 hours. Ital J Anim Sci. 13: 3444.
Faigl V, Vass N, Javor A, Kulcsár M, Solti L, Amiridis G, Cseh S. 2012. Artificial insemination of small ruminants - A review. Acta Vet Hung. 60: 115-29.
Gibbons AE, Fernandez J, Bruno-Galarraga MM, Spinelli MV, Cueto MI. 2019. Technical recommendations for artificial insemination in sheep. Anim Reprod. 16: 803-9.
Ax RL, Dally MR, Didion BA, Lenz RW, Loce CC, Varner DD, Hafez B, Bellin ME. 2000. Artificial insemination. In: Hafez B, Hafez ESE (Eds). Reproduction in Farm Animals. 7th Ed. Lippincott Williams and Wilkins. USA. 451-70.
Halliwell B. 2013. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol. 75: 637-44.
Ismaya. 2014. Biotechnology for artificial insemination in cattle and buffalo. Gadjah MAda University Press. Yogyakarta.
Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. 2013. Tissue damage and oxidant/antioxidant balance. Eurasian J Med. 45: 47-9.
Kumar D, Naqvi SMK. 2014. Effect of time and depth of insemination on the fertility of Bharat Merino sheep inseminated transcervical with frozen-thawed semen. J Anim Sci Technol. 56: 8.
Macías A, Ferrer LM, Ramos JJ, Lidón I, Rebollar R, Lacasta D, Tejedor MT. 2017. Technical Note: A new device for cervical insemination of sheep-design and field test. J Anim Sci. 95: 5263-9.
Mehta SK, Gowder SJT. 2015. Members of antioxidant machinery and their functions. In: Gowder SJT (Ed). Basic principles and clinical significance of oxidative stress. IntechOpen.
Miki K. 2007. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 65: 309-25.
Mudawamah M, Anwar MZ, Sumartono S. 2022. Estimation of repeatability and most probable producing ability (MPPA) of Sapudi sheep based on daily body weight gain of lambs from birth to pre-weaning and weaning. J Sain Peternakan Indonesia 17: 149-54.
Panche AN, Diwan AD, Chandra SR. 2016. Flavonoids: an overview. J Nutr Sci. 5: e47.
Pintus E, Ros-Santaella JL. 2021. Impact of oxidative stress on male reproduction in domestic and wild animals. Antioxidants 10: 1154.
Polat ES, Citil OB, Garip M. 2013. Fatty acid composition of the yolk of nine poultry species kept in their natural environment. Anim Sci Pap Rep. 31: 363-8.
Puspita SAC, Susilowati S, Soeharsono S, Sardjito T, Samik A, Triana IN. 2020. Addition of alpha-tocopherol in skim milk-egg yolk extender on the quality of Sapudi ram spermatozoa stored at 5°C. Ovozoa : J Anim Reprod. 9: 69-76.
Putri CD, Ismudiono, and Poetranto ED. 2021. The Effect of the Different Artificial Insemination Time Periods on the Pregnancy Rate of Sapudi Ewes. World Vet J. 11: 469-473.
Quraini SP, SusilowatiS, ChusniatiS, RestiadiTI. 2022. Comparison of different poultry egg yolks-citrate extenders with green tea (Camellia sinensis) extract addition on Sapudi ram spermatozoa quality in chilled temperature storage. Ovozoa : J Anim Reprod. 11: 93-7.
Retta ATM, Susilowati S, Wahyuni RS, Madyawati SP, Hernawati T, Wurlina W. 2022. Effect of fruit juices in skim milk extender in maintaining Sapudi ram spermatozoa quality at a chilled temperature. Ovozoa : J Anim Reprod. 11: 49-53.
Reynolds S, Ismail NFB, Calvert SJ, Pacey AA, Paley MNJ. 2017. Evidence for rapid oxidative phosphorylation and lactate fermentation in motile human sperm by hyperpolarized 13C magnetic resonance spectroscopy. Sci Rep. 7: 4322.
Rizkallah N, Chambers CG, de Graaf SP, Rickard JP. 2022. Factors affecting the survival of ram spermatozoa during liquid storage and options for improvement. Animals 12: 244.
Roychoudhury S, Agarwal A, Virk G, Cho CL. 2017. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod Biomed Online 34: 487-98.
Simíµes J, Abecia JA, Cannas A, Delgadillo JA, Lacasta D, Voigt K, Chemineau P. 2021. Review: Managing sheep and goats for sustainable high-yield production. Animals 15: 100293.
Susilowati S, Mustofa I, Wurlina W, Hernawatia T, Oktanella Y. 2021. Maintaining the quality of kacang buck semen in chilled storage with the addition of green tea extract in the extender. Trop Anim Sci J. 44: 408-14.
Susilowati S, Triana IN, Wurlina W, Arimbi A, Srianto P, Mustofa I. 2019. The addition of L-arginine in the skim milk extender maintains goat spermatozoa quality at a chilled temperature for five days. Vet World 12: 1784-9.
Swelum AA, Ba-Awadh HA, Olarinre IO, Saadeldin IM, Alowaimer AN. 2022. Effects of adding mixed chicken and quail egg yolks to the cryo-diluent on the quality of ram semen before and after cryopreservation. Front Vet Sci. 9: 1013533.
Tang GY, Meng X, Gan RY, Zhao CN, Liu Q, Feng YB, Li S, Wei XL, Atanasov AG, Corke H, Li HB. 2019. Health functions and related molecular mechanisms of tea components: An update review. Int J Mol Sci. 20: 6196.
Tanga BM, Qamar AY, Raza S, Bang S, Fang X, Yoon K, Cho J. 2021. Semen evaluation: Methodological advancements in sperm quality-specific fertility assessment - A review. Anim Biosci. 34: 1253-1270.
Van de Hoek M, Rickard JP, de Graaf SP. 2022. Motility assessment of ram spermatozoa. Biology 11: 1715.
Vera-Munoz O, Amirat-Briand L, Bencharif D, Anton M, Desherces S, Shmitt E, Thorin C, Tainturier D. 2011. Effect of low-density lipoproteins, spermatozoa concentration, and glycerol on functional and motility parameters of bull spermatozoa during storage at 4 °C. Asian J Androl. 13: 281-6.
Zhang L, Wang Y, Sun X, Kang Y, Sohail T, Wang J, Li Y. 2023. Effects of different diluents on semen quality of Hu ram stored at 4 °C. Animals 13: 2823.
Zhang R, Li X, Fan C, Ning Z. 2022. Effects of lipoproteins on yolk microstructure in duck, quail, goose, pigeon, and chicken eggs. Food Sci Technol. 42: e00222.
Copyright (c) 2023 Ardina Sahra Miranda, Tri Wahyu Suprayogi, Budi Utomo, Suherni Susilowati, Yeni Dhamayanti

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Ovozoa by Unair is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
1. The journal allows the author to hold the copyright of the article without restrictions.
2. The journal allows the author(s) to retain publishing rights without restrictions
3. The legal formal aspect of journal publication accessibility refers to Creative Commons Attribution Share-Alike (CC BY-SA).
4. The Creative Commons Attribution Share-Alike (CC BY-SA) license allows re-distribution and re-use of a licensed work on the conditions that the creator is appropriately credited and that any derivative work is made available under "the same, similar or a compatible license”. Other than the conditions mentioned above, the editorial board is not responsible for copyright violation.