Date Log
Copyright (c) 2023 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
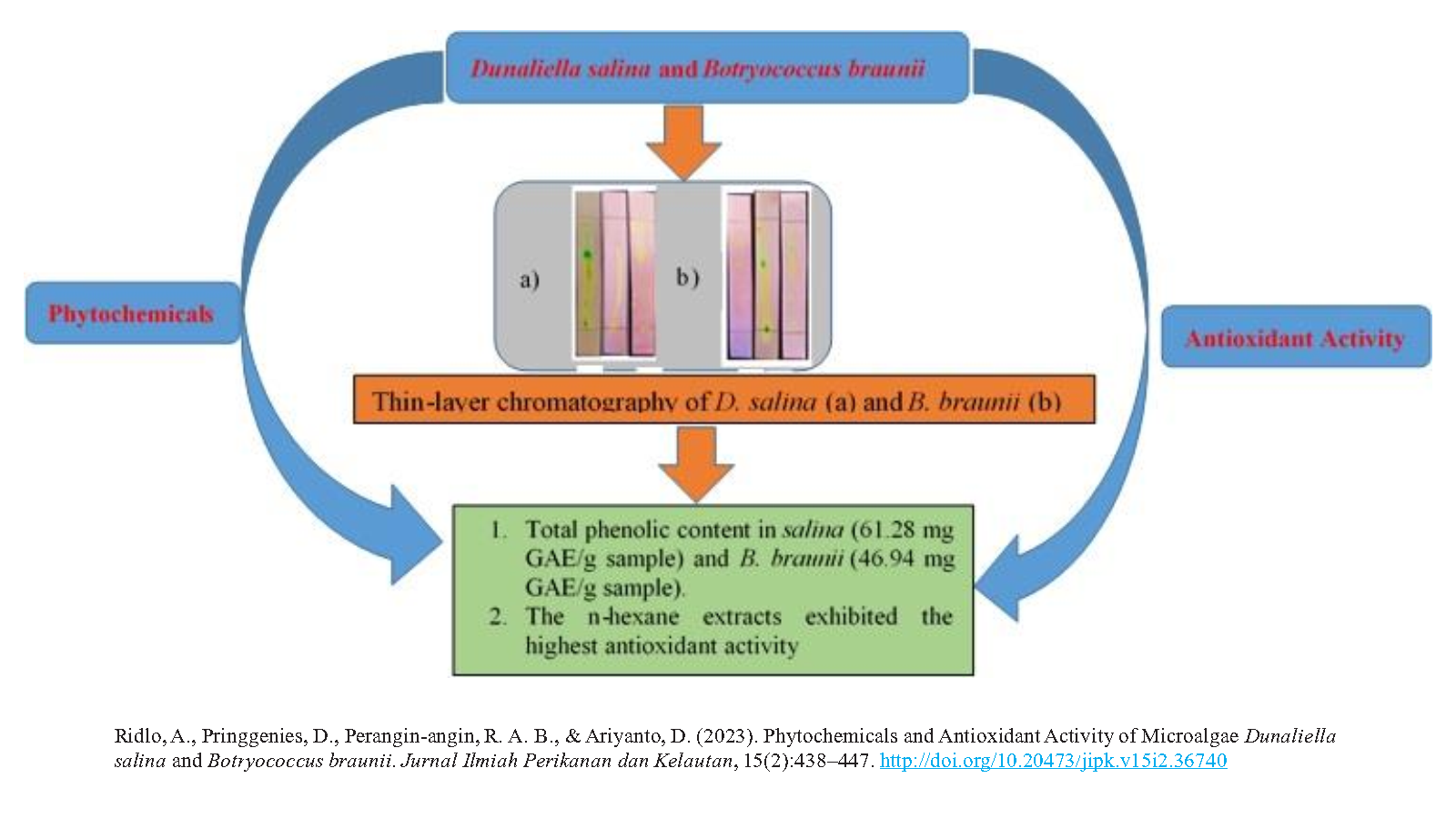
Phytochemicals and Antioxidant Activity of Microalgae Dunaliella salina and Botryococcus braunii
Corresponding Author(s) : Ali Ridlo
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 15 No. 2 (2023): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Highlight Research
- The methanolic extracts of salinaand B. braunii contained alkaloids, steroids, triterpenoids, flavonoids, and saponins.
- Chlorophyll a, chlorophyll b, and carotenoids were most abundant in the ethyl acetate extracts of salinaand B. braunii.
- The maximum total phenolic content was observed in the n-hexane extract of salina(61.28 mg GAE/g sample) and the ethyl acetate extract of B. braunii (46.94 mg GAE/g sample).
- The n-hexane extracts exhibited the highest antioxidant activity, whereas saponins were most abundant in the methanol extracts.
Abstract
Microalgal species such as Dunaliella salina and Botryococcus braunii are reportedly rich in natural antioxidants and phytochemicals. This study aimed to determine the phytochemicals and the antioxidant activity of D. salina and B. braunii. Microalgal samples were obtained from the Brackish Water Cultivation Fisheries Center (BPBAP), Situbondo, East Java. The extracts were prepared using the multilevel maceration method. The antioxidant activity of the algal species was analyzed using 1,1-diphenyl-2-picrylhydraxyl (DPPH). Quantitative analysis revealed that D. salina and B. braunii contained antioxidants, indicated by the appearance of yellow spots on the purple background of the TLC plate. The n-hexane extract of D. salina exhibited the highest antioxidant activity with a half-maximal inhibitory concentration (IC50) of 443.28 ppm, 61.28 mg GAE/g sample of total phenolics, 0.106 mg/g of chlorophyll a, 0.165 mg/g of chlorophyll b, and 1,697 mol/g of carotenoids. In contrast, the ethyl acetate extract of B. braunii exhibited the highest antioxidant activity with an IC50 of 634.55 ppm, 46.94 mg GAE/g sample of total phenolics, 18.146 mg/g of chlorophyll a, 12.592 mg/g of chlorophyll b, and 4573 mol/g of carotenoids. The microalgal species used in this study exhibited extremely weak antioxidant activity.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Akar, Z., Küçük, M., & Doğan, H. (2017). A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. Journal of Enzyme Inhibition and Medicinal Chemistry, 32(1):640-647.
- Akbar, M. R., Pramesti, R., & Ridlo, A., (2018). Antioxidant activity of Acanthophora muscoides (Linnaeus) bory seaweed from Krakal Beach, Gunung Kidul, Yogyakarta. Journal of Marine Research, 7(1):9-18.
- Augustynska, D., JemioÅ‚a-Rzemiå ska, M., Burda, K., & StrzaÅ‚ka, K. (2015). Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chemico-Biological Interactions, 239:19-25.
- Auwal, M. S., Saka, S., Mairiga, I. A., Sanda, K. A., Shuaibu, A., & Ibrahim, A. (2014). Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Veterinary Research Forum: An International Quarterly Journal, 5(2),95-100.
- Badr, A. N., Youssef, M., Abdel-Razek, A. G., Shehata, M. G., Hassanien, M. M., & Amra, H. (2021). Natural antioxidants: Preservation roles and mycotoxicological safety of food. Egyptian Journal of Chemistry, 64(1):285-298.
- Barkia, I., Saari, N., & Manning, S. R. (2019). Microalgae for high-value products towards human health and nutrition. Marine Drugs, 17(5):1–29.
- Blainski, A., Lopes, G. C., & De Mello, J. C. P. (2013). Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules, 18(6):6852-6865.
- Blifernez-Klassen, O., Chaudhari, S., Klassen, V., Wördenweber, R., Steffens, T., Cholewa, D., Niehaus, K., Kalinowski, J., & Kruse, O. (2018). Metabolic survey of Botryococcus braunii: Impact of the physiological state on product formation. PLoS ONE, 13(6):1-23.
- Dai, J., & Mumper, R. J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules, 15(10):7313-7352.
- Dineshkumar, R., Ahamed R. A., Arumugam, A., Nathiga, N. K.S., & Sampathkumar, P. (2019). Marine microalgal extracts on cultivable crops as a considerable bio-fertilizer: A review. Indian Journal of Traditional Knowledge, 18(4):249-254.
- Dragone, G. (2022). Challenges and opportunities to increase economic feasibility and sustainability of mixotrophic cultivation of green microalgae of the genus Chlorella. Renewable and Sustainable Energy Reviews, 160:112284.
- Elalami, D., Oukarroum, A., & Barakat, A. (2021). Anaerobic digestion and agronomic applications of microalgae for its sustainable valorization. RSC Advances, 11:26444.
- Faizal, A., & Geelen, D. (2013). Saponins and their role in biological processes in plants. Phytochemistry Reviews, 12:877-893.
- Fithriani, D., Amini, S., Melanie, S., & Susilowati, R. (2015). Phytochemical screening, total phenol content and antioxidant activity of microalgae Spirulina sp., Chlorella sp. and Nannochloropsis sp. Jurnal Pascapanen dan Bioteknologi Kelautan dan Perikanan, 10(2):101–109.
- Foo, S. C., Yusoff, F. M., Ismail, M., Basri, M., Khong, N. M. H., Chan, K. W., & Yau, S. K. (2015). Efficient solvent extraction of antioxidant-rich extract from a tropical diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Asian Pacific Journal of Tropical Biomedicine, 5(10):834-840.
- Ghasemi, K., Ghasemi, Y., & Ebrahimzadeh, M. A. (2009). Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pakistan Journal of Pharmaceutical Sciences, 22(3):277–281.
- González-Palma, I., Escalona-Buendía, H. B., Ponce-Alquicira, E., Téllez-Téllez, M., Gupta, V. K., Díaz-Godínez, G., & Soriano-Santos, J. (2016). Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Frontiers in Microbiology, 7:1-9.
- Hidayati, M. D., Ersam, T., Shimizu, K., & Fatmawat, S. (2017). Antioxidant activity of Syzygium polyanthum extracts. Indonesian Journal of Chemistry, 7(1):49-53.
- Kalinowska, M., Zimowski, J., PÄ…czkowski, C., & Wojciechowski, Z. A. (2005). The formation of sugar chains in triterpenoid saponins and glycoalkaloids. Phytochemistry Reviews, 4:237-257.
- Koyande, A. K., Chew, K. W., Rambabu, K., Tao, Y., Chu, D. T., & Show, P. L. (2019). Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness, 8(1):16-24.
- Kumar, S., & Pandey, A. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 58:1-17.
- Kurniasih, S. D., Pramesti, R., & Ridlo, A. (2014). Determination of antioxidant activity of seaweed extract Ulva sp. from Krakal Beach, Yogyakarta. Diponegoro. Journal of Marine Research, 3(4):617-626.
- Munteanu, I. G., & Apetrei, C. (2021). Analytical methods used in determining antioxidant activity: A review. International Journal of Molecular Sciences, 22(3380):1-30.
- Muthia, R., Saputri, R., & Verawati, S. A. (2019). Antioxidant activity test of ethanol extract of mundar fruit rind (Garcinia forbesii King.) using method DPPH (2,2-Diphenyl-1-Picrylhydrazil). Journal Pharmascience, 6(1):74-82.
- Podolak, I., Galanty, A., & Sobolewska, D. (2010). Saponins as cytotoxic agents: A review. Phytochemistry Reviews, 9:425-474.
- Prayitno, D. I, Dewi E. N., Pringgenies, D., & Brotosudarmo, T. H. P. (2022) Green ultrasound-assisted extraction of astaxanthin from fermented rebon shrimp (cincalok) using vegetable oils as solvents. Oilseeds & fats Crops and Lipids, 29(15):1-8.
- Prasad, R., Gupta, S. K., Shabnam, N., Oliveira, C. Y. B., Nema, A. K., Ansari, F. A., & Bux, F. (2021). Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability, 13(13061):1–18.
- Pujiyanto, M., Afililla, Z., Maslachah, L., Widiyatno, T. V., Koerniawan, M. D., Suyono, E. A., Budiman, A., Siregar, U. J., & Suwanti, L. T. (2022). The activity of mixed microalgae Polysaccharides from Indonesia as anti-malaria in vitro. Jurnal Ilmiah Perikanan dan Kelautan, 14(2):396–403.
- Ridlo, A., Sedjati, S., & Supriyantini, E. (2015). Anti-oxidant activity of phycocyanin from Spirulina sp. using the electron transfer method with DPPH (1,1-difenil-2-pikrilhidrazil). Jurnal Kelautan Tropis, 18(2):58-63.
- Saadaoui, I., Rasheed, R., Aguilar, A., Cherif, M., Al Jabri, H., Sayadi, S., & Manning, S. R. (2021). Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. Journal of Animal Science and Biotechnology, 12(1):1–15.
- Salvador, M. J., De Lourenço, C. C., Andreazza, N. L., Pascoal, A. C. R. F., & Stefanello, M. í‰. A. (2011). Antioxidant capacity and phenolic content of four myrtaceae plants of the South of Brazil. Natural Product Communications, 6(7):977-982.
- Setha, B., Gaspersz, F. F., Idris, A. P. S., Rahman, S., & Mailoa, M. N. (2013). Potential of seaweed Padina sp. as a source of antioxidant. International Journal of Scientific & Technology Research, 2(6):221-224.
- Singh, P., Baranwal, M., & Reddy, S. M. (2016). Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharmaceutical Biology, 54(10):2269-2275.
- Stunda-Zujeva, A., Zuteris, M., & Rugele, K. (2018). Sunlight potential for microalgae cultivation in the mid-latitude region the Baltic states. Agronomy Research, 16(3):910–916.
- Trentin, R., Jo, M., Moschin, E., Sciuto, K., & Moro, I. (2022). Total phenolic levels, in vitro antioxidant properties, and fatty IMA043 and naviculoid diatom strain IMA053, isolated from the north Adriatic Sea. Marine Drugs, 20(207):1-17.
- Vaz, B. da S., Moreira, J. B., Morais, M. G. de, & Costa, J. A. V. (2016). Microalgae as a new source of bioactive compounds in food supplements. Current Opinion in Food Science, 7:73-77.
- Xu, Y., & Harvey, P. J. (2019). Carotenoid production by Dunaliella salina under red light. Antioxidants, 8(123):1-14.
- Zainuddin, M. (2017). Antioxidant activity of Dunaliella salina biopigment in hyposline and hypersaline cultures. Journal Enggano, 2(1):25-38.
- Zeb, A., Haq, A., & Murkovic, M. (2019). Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (Chicory) leaves. European Food Research and Technology, 245:1-10.
- Zielewicz, W., Wróbel, B., & NiedbaÅ‚a, G. (2020). Quantification of chlorophyll and carotene pigments content in mountain melick (Melica nutans L.) in relation to edaphic variables. Forests, 11(11):1-16.
References
Akar, Z., Küçük, M., & Doğan, H. (2017). A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. Journal of Enzyme Inhibition and Medicinal Chemistry, 32(1):640-647.
Akbar, M. R., Pramesti, R., & Ridlo, A., (2018). Antioxidant activity of Acanthophora muscoides (Linnaeus) bory seaweed from Krakal Beach, Gunung Kidul, Yogyakarta. Journal of Marine Research, 7(1):9-18.
Augustynska, D., JemioÅ‚a-Rzemiå ska, M., Burda, K., & StrzaÅ‚ka, K. (2015). Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chemico-Biological Interactions, 239:19-25.
Auwal, M. S., Saka, S., Mairiga, I. A., Sanda, K. A., Shuaibu, A., & Ibrahim, A. (2014). Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Veterinary Research Forum: An International Quarterly Journal, 5(2),95-100.
Badr, A. N., Youssef, M., Abdel-Razek, A. G., Shehata, M. G., Hassanien, M. M., & Amra, H. (2021). Natural antioxidants: Preservation roles and mycotoxicological safety of food. Egyptian Journal of Chemistry, 64(1):285-298.
Barkia, I., Saari, N., & Manning, S. R. (2019). Microalgae for high-value products towards human health and nutrition. Marine Drugs, 17(5):1–29.
Blainski, A., Lopes, G. C., & De Mello, J. C. P. (2013). Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules, 18(6):6852-6865.
Blifernez-Klassen, O., Chaudhari, S., Klassen, V., Wördenweber, R., Steffens, T., Cholewa, D., Niehaus, K., Kalinowski, J., & Kruse, O. (2018). Metabolic survey of Botryococcus braunii: Impact of the physiological state on product formation. PLoS ONE, 13(6):1-23.
Dai, J., & Mumper, R. J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules, 15(10):7313-7352.
Dineshkumar, R., Ahamed R. A., Arumugam, A., Nathiga, N. K.S., & Sampathkumar, P. (2019). Marine microalgal extracts on cultivable crops as a considerable bio-fertilizer: A review. Indian Journal of Traditional Knowledge, 18(4):249-254.
Dragone, G. (2022). Challenges and opportunities to increase economic feasibility and sustainability of mixotrophic cultivation of green microalgae of the genus Chlorella. Renewable and Sustainable Energy Reviews, 160:112284.
Elalami, D., Oukarroum, A., & Barakat, A. (2021). Anaerobic digestion and agronomic applications of microalgae for its sustainable valorization. RSC Advances, 11:26444.
Faizal, A., & Geelen, D. (2013). Saponins and their role in biological processes in plants. Phytochemistry Reviews, 12:877-893.
Fithriani, D., Amini, S., Melanie, S., & Susilowati, R. (2015). Phytochemical screening, total phenol content and antioxidant activity of microalgae Spirulina sp., Chlorella sp. and Nannochloropsis sp. Jurnal Pascapanen dan Bioteknologi Kelautan dan Perikanan, 10(2):101–109.
Foo, S. C., Yusoff, F. M., Ismail, M., Basri, M., Khong, N. M. H., Chan, K. W., & Yau, S. K. (2015). Efficient solvent extraction of antioxidant-rich extract from a tropical diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Asian Pacific Journal of Tropical Biomedicine, 5(10):834-840.
Ghasemi, K., Ghasemi, Y., & Ebrahimzadeh, M. A. (2009). Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pakistan Journal of Pharmaceutical Sciences, 22(3):277–281.
González-Palma, I., Escalona-Buendía, H. B., Ponce-Alquicira, E., Téllez-Téllez, M., Gupta, V. K., Díaz-Godínez, G., & Soriano-Santos, J. (2016). Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Frontiers in Microbiology, 7:1-9.
Hidayati, M. D., Ersam, T., Shimizu, K., & Fatmawat, S. (2017). Antioxidant activity of Syzygium polyanthum extracts. Indonesian Journal of Chemistry, 7(1):49-53.
Kalinowska, M., Zimowski, J., PÄ…czkowski, C., & Wojciechowski, Z. A. (2005). The formation of sugar chains in triterpenoid saponins and glycoalkaloids. Phytochemistry Reviews, 4:237-257.
Koyande, A. K., Chew, K. W., Rambabu, K., Tao, Y., Chu, D. T., & Show, P. L. (2019). Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness, 8(1):16-24.
Kumar, S., & Pandey, A. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 58:1-17.
Kurniasih, S. D., Pramesti, R., & Ridlo, A. (2014). Determination of antioxidant activity of seaweed extract Ulva sp. from Krakal Beach, Yogyakarta. Diponegoro. Journal of Marine Research, 3(4):617-626.
Munteanu, I. G., & Apetrei, C. (2021). Analytical methods used in determining antioxidant activity: A review. International Journal of Molecular Sciences, 22(3380):1-30.
Muthia, R., Saputri, R., & Verawati, S. A. (2019). Antioxidant activity test of ethanol extract of mundar fruit rind (Garcinia forbesii King.) using method DPPH (2,2-Diphenyl-1-Picrylhydrazil). Journal Pharmascience, 6(1):74-82.
Podolak, I., Galanty, A., & Sobolewska, D. (2010). Saponins as cytotoxic agents: A review. Phytochemistry Reviews, 9:425-474.
Prayitno, D. I, Dewi E. N., Pringgenies, D., & Brotosudarmo, T. H. P. (2022) Green ultrasound-assisted extraction of astaxanthin from fermented rebon shrimp (cincalok) using vegetable oils as solvents. Oilseeds & fats Crops and Lipids, 29(15):1-8.
Prasad, R., Gupta, S. K., Shabnam, N., Oliveira, C. Y. B., Nema, A. K., Ansari, F. A., & Bux, F. (2021). Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability, 13(13061):1–18.
Pujiyanto, M., Afililla, Z., Maslachah, L., Widiyatno, T. V., Koerniawan, M. D., Suyono, E. A., Budiman, A., Siregar, U. J., & Suwanti, L. T. (2022). The activity of mixed microalgae Polysaccharides from Indonesia as anti-malaria in vitro. Jurnal Ilmiah Perikanan dan Kelautan, 14(2):396–403.
Ridlo, A., Sedjati, S., & Supriyantini, E. (2015). Anti-oxidant activity of phycocyanin from Spirulina sp. using the electron transfer method with DPPH (1,1-difenil-2-pikrilhidrazil). Jurnal Kelautan Tropis, 18(2):58-63.
Saadaoui, I., Rasheed, R., Aguilar, A., Cherif, M., Al Jabri, H., Sayadi, S., & Manning, S. R. (2021). Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. Journal of Animal Science and Biotechnology, 12(1):1–15.
Salvador, M. J., De Lourenço, C. C., Andreazza, N. L., Pascoal, A. C. R. F., & Stefanello, M. í‰. A. (2011). Antioxidant capacity and phenolic content of four myrtaceae plants of the South of Brazil. Natural Product Communications, 6(7):977-982.
Setha, B., Gaspersz, F. F., Idris, A. P. S., Rahman, S., & Mailoa, M. N. (2013). Potential of seaweed Padina sp. as a source of antioxidant. International Journal of Scientific & Technology Research, 2(6):221-224.
Singh, P., Baranwal, M., & Reddy, S. M. (2016). Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharmaceutical Biology, 54(10):2269-2275.
Stunda-Zujeva, A., Zuteris, M., & Rugele, K. (2018). Sunlight potential for microalgae cultivation in the mid-latitude region the Baltic states. Agronomy Research, 16(3):910–916.
Trentin, R., Jo, M., Moschin, E., Sciuto, K., & Moro, I. (2022). Total phenolic levels, in vitro antioxidant properties, and fatty IMA043 and naviculoid diatom strain IMA053, isolated from the north Adriatic Sea. Marine Drugs, 20(207):1-17.
Vaz, B. da S., Moreira, J. B., Morais, M. G. de, & Costa, J. A. V. (2016). Microalgae as a new source of bioactive compounds in food supplements. Current Opinion in Food Science, 7:73-77.
Xu, Y., & Harvey, P. J. (2019). Carotenoid production by Dunaliella salina under red light. Antioxidants, 8(123):1-14.
Zainuddin, M. (2017). Antioxidant activity of Dunaliella salina biopigment in hyposline and hypersaline cultures. Journal Enggano, 2(1):25-38.
Zeb, A., Haq, A., & Murkovic, M. (2019). Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (Chicory) leaves. European Food Research and Technology, 245:1-10.
Zielewicz, W., Wróbel, B., & NiedbaÅ‚a, G. (2020). Quantification of chlorophyll and carotene pigments content in mountain melick (Melica nutans L.) in relation to edaphic variables. Forests, 11(11):1-16.