Date Log
Copyright (c) 2023 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
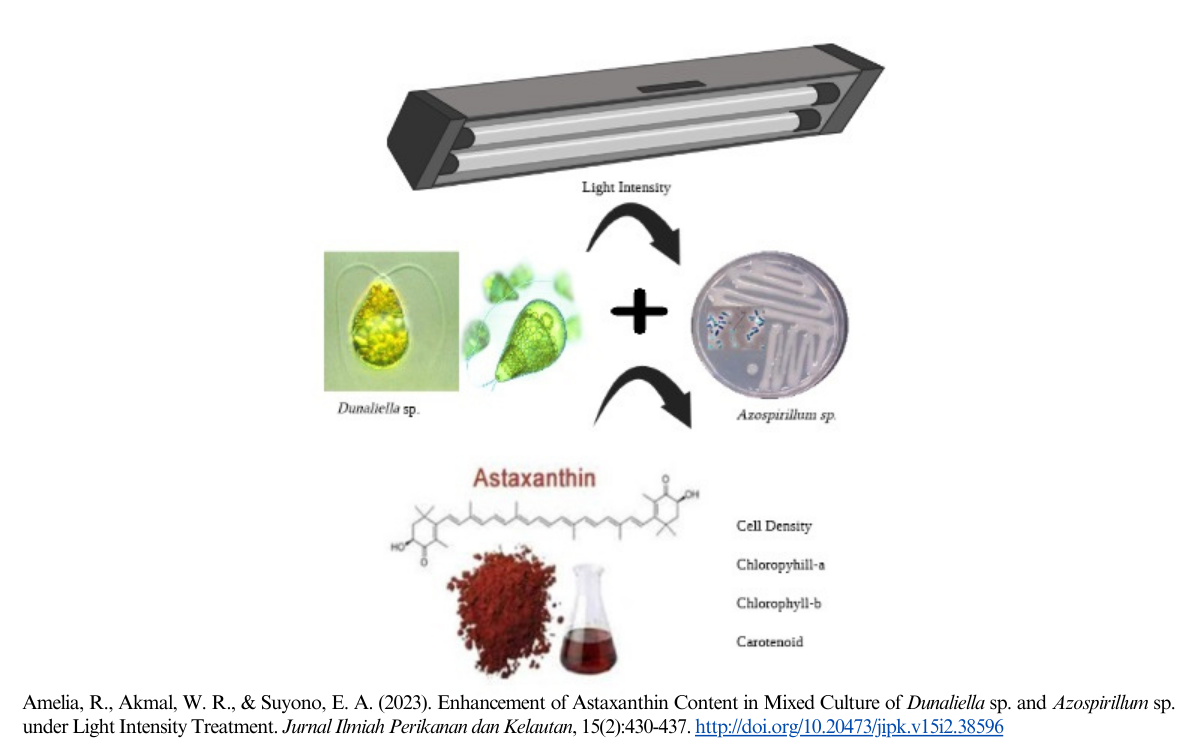
Enhancement of Astaxanthin Content in Mixed Culture of Dunaliella sp. and Azospirillum sp. under Light Intensity Treatment
Corresponding Author(s) : Eko Agus Suyono
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 15 No. 2 (2023): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Highlight Research
- The growth rate was higher when Chlorella was induced by Azospirillum brasilense.
- The carbohydrate content increased when combined with Azospirillum brasilense.
- Determination of astaxanthin accumulation in Haematococcus pluvialis.
- Define condition favoring astaxanthin accumulation in Haematococcus pluvialis.
Abstract
Dunaliella sp. is a potential natural source of carotenoid pigments such as astaxanthin, β-carotene, and lutein. Dunaliella sp. can also accumulate other valuable products such as glycerol and protein. Another species is Azospirillum sp., which is known as microalgal growth-promoting bacteria. These bacteria are often cultured with microalgae because they contain indole-3-acetic acid, which can significantly increase the growth of microalgae. This study aimed to examine the pigment content in mixed culture of Dunaliella sp. and Azospirillum sp. after being treated with different light intensity treatment. In this study, Dunaliella sp. were cultivated by mixing with Azospirillum sp. under light stress. Two treatments were performed at light stress intensity of 3000 and 6000 lx. Light intensity is widely used as an important parameter in cultivation, which can affect the growth and production of microalgal biomass. In addition, spectrophotometric UV-Vis based measurement was conducted to investigate every single pigment content in all treatments under light stress for eight days. The number of cells, carotenoid pigments, and astaxanthin had increased significantly. Pigments of chlorophyll a and chlorophyll b also significantly increased at lower light treatments. Based on the results, the bacterium Azospirillum sp. and high light intensity significantly increased the growth and cell division of microalgae. Therefore, the combination of Azospirillum sp. and light stress intensity in microalgae cultivation could increase the growth and pigment of Dunaliella sp.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Amelia, R. (2018). Pengaruh intensitas cahaya terhadap kandungan pigmen kultur konsorsium Dunaliella sp. dan Azospirillum sp. Yogyakarta: Universitas Gadjah Mada.
- Bashan, Y., & de-Bashan, L. E. (2010). How the plant growth-promoting bacterium Azospirillum promotes plant growth-A critical assessment. Advances in Agronomy, 108:77-136.
- Boussiba, S., Fan, L., & dan Vonshak, A. (1992). Enhancement dan determination of Astaxanthin accumulation in green alga Haematococcus pluvialis. Methods in Enzymology, 213:386-391.
- Cassán, F. D., Lucangeli, C. D., Bottini, R., & Piccoli, P. N. (2001). Azospirillum spp. metabolize [17, 17- 2 H2] gibeberalin A20 to [17, 17-2H 2] gibberalin A1 in vivo in dy rice mutant seedlings. Plant and Cell Physiology, 42(7):763-767.
- Cazzaniga, S., Perozeni, F., Baier, T., & Ballottari, M. (2022). Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnology for Biofuels and Bioproduct, 15(1):1-17.
- Chen, M. (2014). Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annual Review of Biochemistry, 83:317-340.
- Chen, Y., Bi, C., Zhang, J., Hou, H., & Gong, Z. (2020). Astaxanthin biosynthesis in transgenic Dunaliella salina (Chlorophyceae) enhanced tolerance to high irradiation stress. South African Journal of Botany, 133:132-138.
- Chisti, Y. (2013). Constraints to commercialization of algal fuels. Journal of Biotechnology, 167(3):201-214.
- Choix, F. J., de-Bashan, L. E., & Bashan, Y. (2012). Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzyme and Microbial Technology, 51(5):294-299.
- Coates, R. C., Trentacoste, E., & Gerwick, W. H. (2013). Bioactive and novel chemicals from microalgae. In A. Richmond, Emeritus, and Q. Hu (Ed.), Handbook of microalgal culture. (pp. 504-531). New York: John Wiley & Sons.
- Contreras-Angulo, J., Mata, T. M., Cuellar-Bermudez. S. P., Caetano, N. S., Chandra, R., Garcia-Perez, J. S., Muylaert, K., & Parra-Saldivar, R. (2019). Symbiotic co-culture of Scenedesmus sp. and Azospirillum brasilense on n-deficient media with biomass production for biofuels. Sustainability, 11(707):1-16.
- da Silva, J. C., & Lambordi, A. T. (2020). Chlorophylls in microalgae: occurrence, distribution, and biosynthesis. In E Jacob-Lopes, M. Queiroz, L. Zepka (Ed.), Pigments from microalgae handbook. (pp. 1-18). Switzerland: Springer, Cham.
- Darley, W. M. (1982) Algal biology: A physiological approach. Chapter 3: Phytoplankton: environmental factors affecting growth. Boston: Blackwell Scientific Publications.
- Demming-Adams, B., & Adams, W. W. (2002). Antioxidants in photosynthesis and human nutrition. Science, 298(5601):2149-2153.
- Elfiza, W. N., Dharma, A., & Nasir, N. (2019). Penapisan mikroalga penghasil karotenoid serta studi pengaruh stres nitrogen and fosfor terhadap produksi B-karoten pada mikroalga Oocytis sp. Jurnal Pasca Panen dan Bioteknologi Kelautan dan Perikanan, 14(1):9-20.
- Ferreira, V. S., Pinto, R. F., & Sant'Anna, C. (2015). Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. Journal of Applied Microbiology, 120(3):661-670.
- Guedes, A. C., Meireles, L. A., Amaro, H. M., & Malcata, F. X. (2010). Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. Journal of the American Oil Chemists Society, 87(7):791-801.
- Hannon, M., Gimpel, J., Tran, M., Rasala, B., & Mayfield, S. (2010). Biofuels from algae: Challenges and potential. Biofuels, 1(5):763-784.
- Katam, K., Ananthula, R., Anumala, S., Sriariyanun, M., & Bhattacharyya, D. (2022). The impact of light intensity and wavelength on the performance of algal-bacterial culture treating domestic wastewater. E3S Web of Conferences, 355(02003):1-9.
- Mandal, M. K., Chanu, Ng. K., & Chaurasia, N. (2020). Exogenous addition of indole acetic acid and kinetin under nitrogen-limited medium enhances lipid yield and expression of glycerol-3-phosphate acyltransferase & diacylglycerol acyltransferase genes in indigenous microalgae: A potential approach for biodiesel production. Bioresource Technology, 297(1):122439.
- Nick, S., Meurer, J., Soll, J., & Ankele, E. (2013). Nucleus-encoded light-harvesting Chlorophyll a/b proteins are imported normally into Chlorophyll b-Free chloroplasts of Arabidopsis. Molecular Plant, 6(3):860-871.
- Olasehinde, T. A., Olaniran, A. O., & Okoh, A. I. (2017). Therapeutic Potentials of microalgae in the treatment of Alzheimer's disease. Molecules, 22(480):1-18.
- Patil, A D., Kasabe, P. J. & Dandge, P. B. (2022). Pharmaceutical and nutraceutical potential of natural bioactive pigment: Astaxanthin. Natural Products and Bioprospecting, 12(25):1-26.
- Peri, P. L., Pastur, G. M., & Lencinas, M. V. (2009). Photosynthetic responses to different light intensities and water status of two main Nothofagus species of Southern Patagonian Forest, Argentina. Forest Sciences, 55(3):101-111.
- Prawira-Atmaja, M., Shabri., Khomaini, H. S., Maulana, H., Harianto, S., & Rohdiana, D. (2018). Changes in Chlorophyll and Polyphenols content in Camellia sinensis var. sinensis at different stage of leaf maturity. IOP Conference Series: Earth and Environmental Science, 131(012010):1-8.
- Pruvost, J., Vooren, G. V., Le Gouic, B., Massion, A. C., & Legrand, J. (2011). Systematic investigation of biomass and lipid productivity by in photobioreactors for biodiesel application. Bioresource Technology, 102(1):150-158.
- Ramos-Ibarra, J. R., Rubio-Ramírez, T. E., Mondragón-Cortez, P., Torres-Velázquez, J. R., & Choix, F. J. (2019). Azospirillum brasilense-microalga interaction increases growth and accumulation of cell and Tetradesmus obliquus cultures under nitrogen stress. Journal of Applied Phycology, 31(6):3465-3477.
- Silva, M., Farah, K., Uota, S. T., Kovan, I. M., Viegas, C. S. B., Simes, D. C., Gangadhar, K. N., Varela, J. & Barreira, L. (2022). Microalgae as Potential sources of bioactive compounds for functional foods and pharmaceuticals. Applied Sciences, 12(5877):1-26.
- Suyono, E. A., Nopitasari, S., Zusron, M., Khoirunnisa, P., Islami, D. A., & Prabeswara, C. B. (2016). Effect of silica on carbohydrate content of mixed culture Phaedactylum sp. and Chlorella sp. Biosciences Biotechnology Research Asia, 13(1):109-114.
- Tafreshi, A. H., & Shariati, M. (2009). Dunaliella biotechnology: methods and applications. Journal of Applied Microbiology, 107(1):14-35.
- Voitsekhovskaja, O. V., & Tyutereva, E. V. (2015). Chlorophyll b in angiosperms: functions in photosynthesis, signaling and ontogenetic regulation. Journal of Plant Physiology, 189:51-64.
References
Amelia, R. (2018). Pengaruh intensitas cahaya terhadap kandungan pigmen kultur konsorsium Dunaliella sp. dan Azospirillum sp. Yogyakarta: Universitas Gadjah Mada.
Bashan, Y., & de-Bashan, L. E. (2010). How the plant growth-promoting bacterium Azospirillum promotes plant growth-A critical assessment. Advances in Agronomy, 108:77-136.
Boussiba, S., Fan, L., & dan Vonshak, A. (1992). Enhancement dan determination of Astaxanthin accumulation in green alga Haematococcus pluvialis. Methods in Enzymology, 213:386-391.
Cassán, F. D., Lucangeli, C. D., Bottini, R., & Piccoli, P. N. (2001). Azospirillum spp. metabolize [17, 17- 2 H2] gibeberalin A20 to [17, 17-2H 2] gibberalin A1 in vivo in dy rice mutant seedlings. Plant and Cell Physiology, 42(7):763-767.
Cazzaniga, S., Perozeni, F., Baier, T., & Ballottari, M. (2022). Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnology for Biofuels and Bioproduct, 15(1):1-17.
Chen, M. (2014). Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annual Review of Biochemistry, 83:317-340.
Chen, Y., Bi, C., Zhang, J., Hou, H., & Gong, Z. (2020). Astaxanthin biosynthesis in transgenic Dunaliella salina (Chlorophyceae) enhanced tolerance to high irradiation stress. South African Journal of Botany, 133:132-138.
Chisti, Y. (2013). Constraints to commercialization of algal fuels. Journal of Biotechnology, 167(3):201-214.
Choix, F. J., de-Bashan, L. E., & Bashan, Y. (2012). Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzyme and Microbial Technology, 51(5):294-299.
Coates, R. C., Trentacoste, E., & Gerwick, W. H. (2013). Bioactive and novel chemicals from microalgae. In A. Richmond, Emeritus, and Q. Hu (Ed.), Handbook of microalgal culture. (pp. 504-531). New York: John Wiley & Sons.
Contreras-Angulo, J., Mata, T. M., Cuellar-Bermudez. S. P., Caetano, N. S., Chandra, R., Garcia-Perez, J. S., Muylaert, K., & Parra-Saldivar, R. (2019). Symbiotic co-culture of Scenedesmus sp. and Azospirillum brasilense on n-deficient media with biomass production for biofuels. Sustainability, 11(707):1-16.
da Silva, J. C., & Lambordi, A. T. (2020). Chlorophylls in microalgae: occurrence, distribution, and biosynthesis. In E Jacob-Lopes, M. Queiroz, L. Zepka (Ed.), Pigments from microalgae handbook. (pp. 1-18). Switzerland: Springer, Cham.
Darley, W. M. (1982) Algal biology: A physiological approach. Chapter 3: Phytoplankton: environmental factors affecting growth. Boston: Blackwell Scientific Publications.
Demming-Adams, B., & Adams, W. W. (2002). Antioxidants in photosynthesis and human nutrition. Science, 298(5601):2149-2153.
Elfiza, W. N., Dharma, A., & Nasir, N. (2019). Penapisan mikroalga penghasil karotenoid serta studi pengaruh stres nitrogen and fosfor terhadap produksi B-karoten pada mikroalga Oocytis sp. Jurnal Pasca Panen dan Bioteknologi Kelautan dan Perikanan, 14(1):9-20.
Ferreira, V. S., Pinto, R. F., & Sant'Anna, C. (2015). Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. Journal of Applied Microbiology, 120(3):661-670.
Guedes, A. C., Meireles, L. A., Amaro, H. M., & Malcata, F. X. (2010). Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. Journal of the American Oil Chemists Society, 87(7):791-801.
Hannon, M., Gimpel, J., Tran, M., Rasala, B., & Mayfield, S. (2010). Biofuels from algae: Challenges and potential. Biofuels, 1(5):763-784.
Katam, K., Ananthula, R., Anumala, S., Sriariyanun, M., & Bhattacharyya, D. (2022). The impact of light intensity and wavelength on the performance of algal-bacterial culture treating domestic wastewater. E3S Web of Conferences, 355(02003):1-9.
Mandal, M. K., Chanu, Ng. K., & Chaurasia, N. (2020). Exogenous addition of indole acetic acid and kinetin under nitrogen-limited medium enhances lipid yield and expression of glycerol-3-phosphate acyltransferase & diacylglycerol acyltransferase genes in indigenous microalgae: A potential approach for biodiesel production. Bioresource Technology, 297(1):122439.
Nick, S., Meurer, J., Soll, J., & Ankele, E. (2013). Nucleus-encoded light-harvesting Chlorophyll a/b proteins are imported normally into Chlorophyll b-Free chloroplasts of Arabidopsis. Molecular Plant, 6(3):860-871.
Olasehinde, T. A., Olaniran, A. O., & Okoh, A. I. (2017). Therapeutic Potentials of microalgae in the treatment of Alzheimer's disease. Molecules, 22(480):1-18.
Patil, A D., Kasabe, P. J. & Dandge, P. B. (2022). Pharmaceutical and nutraceutical potential of natural bioactive pigment: Astaxanthin. Natural Products and Bioprospecting, 12(25):1-26.
Peri, P. L., Pastur, G. M., & Lencinas, M. V. (2009). Photosynthetic responses to different light intensities and water status of two main Nothofagus species of Southern Patagonian Forest, Argentina. Forest Sciences, 55(3):101-111.
Prawira-Atmaja, M., Shabri., Khomaini, H. S., Maulana, H., Harianto, S., & Rohdiana, D. (2018). Changes in Chlorophyll and Polyphenols content in Camellia sinensis var. sinensis at different stage of leaf maturity. IOP Conference Series: Earth and Environmental Science, 131(012010):1-8.
Pruvost, J., Vooren, G. V., Le Gouic, B., Massion, A. C., & Legrand, J. (2011). Systematic investigation of biomass and lipid productivity by in photobioreactors for biodiesel application. Bioresource Technology, 102(1):150-158.
Ramos-Ibarra, J. R., Rubio-Ramírez, T. E., Mondragón-Cortez, P., Torres-Velázquez, J. R., & Choix, F. J. (2019). Azospirillum brasilense-microalga interaction increases growth and accumulation of cell and Tetradesmus obliquus cultures under nitrogen stress. Journal of Applied Phycology, 31(6):3465-3477.
Silva, M., Farah, K., Uota, S. T., Kovan, I. M., Viegas, C. S. B., Simes, D. C., Gangadhar, K. N., Varela, J. & Barreira, L. (2022). Microalgae as Potential sources of bioactive compounds for functional foods and pharmaceuticals. Applied Sciences, 12(5877):1-26.
Suyono, E. A., Nopitasari, S., Zusron, M., Khoirunnisa, P., Islami, D. A., & Prabeswara, C. B. (2016). Effect of silica on carbohydrate content of mixed culture Phaedactylum sp. and Chlorella sp. Biosciences Biotechnology Research Asia, 13(1):109-114.
Tafreshi, A. H., & Shariati, M. (2009). Dunaliella biotechnology: methods and applications. Journal of Applied Microbiology, 107(1):14-35.
Voitsekhovskaja, O. V., & Tyutereva, E. V. (2015). Chlorophyll b in angiosperms: functions in photosynthesis, signaling and ontogenetic regulation. Journal of Plant Physiology, 189:51-64.