Date Log
Copyright (c) 2023 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
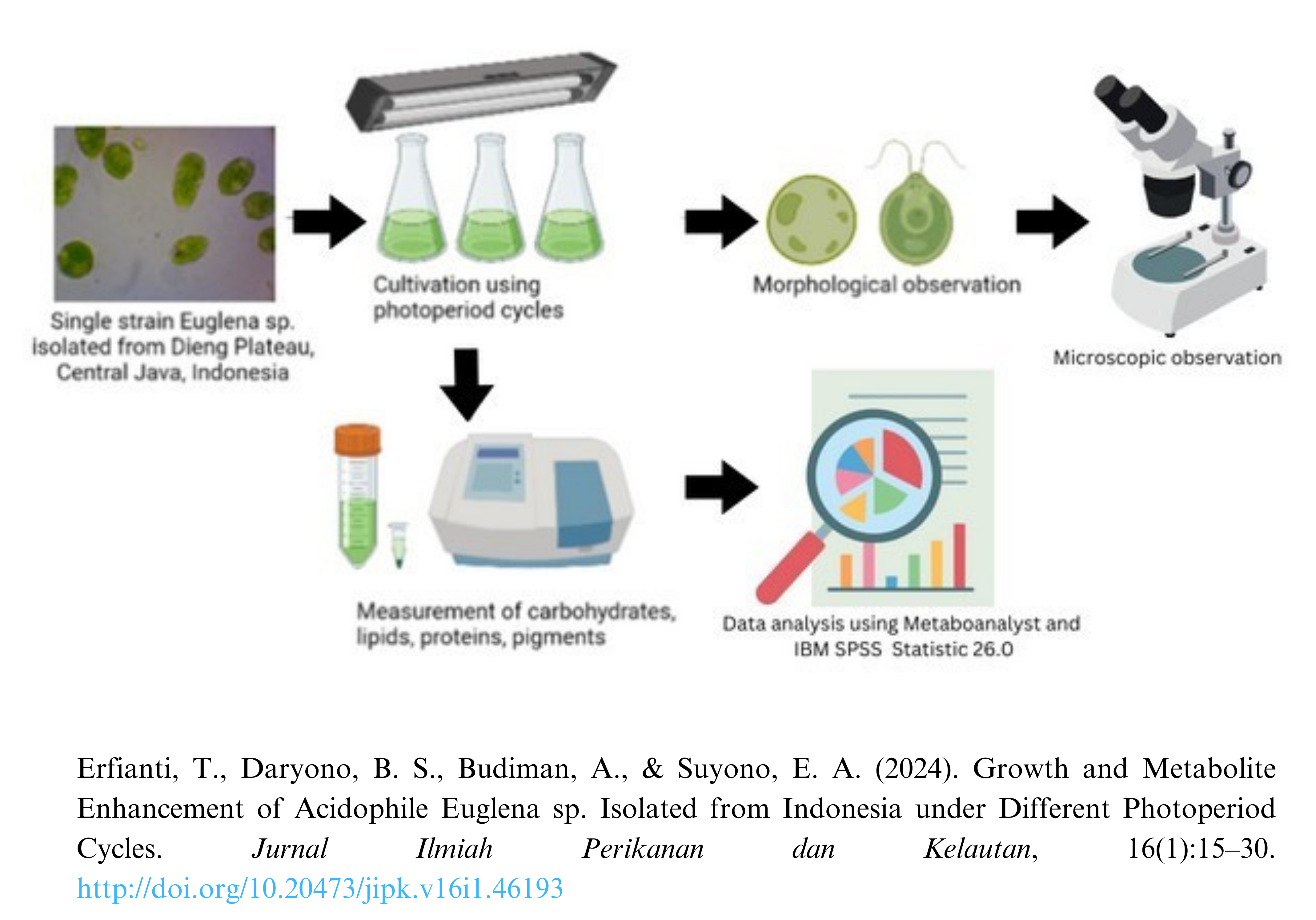
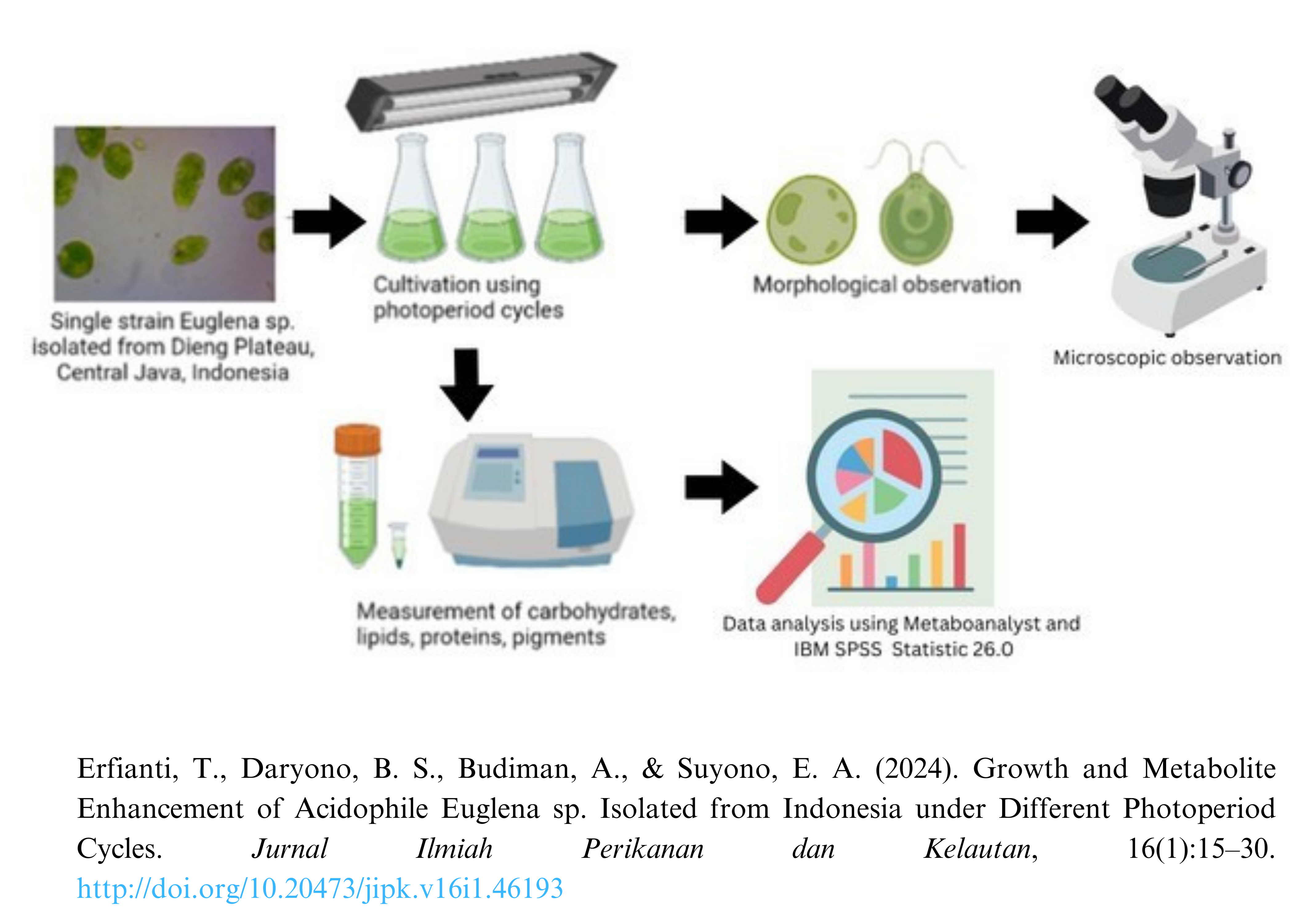
Growth and Metabolite Enhancement of Acidophile Euglena sp. Isolated from Indonesia under Different Photoperiod Cycles
Corresponding Author(s) : Eko Agus Suyono
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 16 No. 1 (2024): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Abstract
Euglena sp. is a unicellular, flagellated microalga considered one of the most promising microalgal feedstock species for biofuels. Reducing the level of liquid waste pollutants can be done biologically by using microalgal organisms. Its metabolites, including lipids, proteins, carbohydrates, and pigments, are appropriate for producing biorefinery products such as biodiesel and jet fuels. They can be isolated from extreme environments, such as highly acidic and ammonia-rich environments, that are not conducive to their proliferation. This study sought to determine the effect of the photoperiod or (light: dark) cycle (24 L:0 D, 12 L:12 D, 14 L:10 D, and 16 L:8 D) on the growth, biomass, metabolite content consisting of lipids, carbohydrates, and proteins, and the rate of CO2 uptake by Euglena sp. As stated previously, the study was conducted by cultivating Euglena sp. on a laboratory scale with four photoperiod regimens. The results indicated that optimal growth, biomass content, and metabolite content were obtained with a 24 D:0 L lighting cycle. The control treatment (24 L: 0 D) had the highest biomass productivity (0.032 g.L-1.day-1 ± 0.004), lipid content (0.387 g.L-1 ± 0.031), protein content (0.542 mg.Ml-1 ± 0.007), carbohydrate content (0.409 x104 g.L-1), chlorophyll a (6.237 g.L-1 ± 0.184), chlorophyll b (2.838 g.L-1 ± 0.253), and total carotenoid (1.566 g.L-1 ± 0.105). Full light illumination (24 L:0 D) was significantly producing carotenoid content, including phaeophytin a, phaeophytin b, violaxanthin, 9'-cis-neoxanthin, dino xanthin, and fucoxanthin.
Highlight Research
- The growth rate was higher when Euglena was cultivated under continuous illumination.
- The biomass productivity of Euglena increased significantly under continuous illumination.
- The metabolite content of Euglena (lipids, proteins, and pigments) was higher in continuous illumination.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Al-Ashra, M S., Abiad, M., & Allahem, A. (2014). Morphological and molecular taxonomical study of Euglena viridis ehren and Euglena gracilis klebs growing in Aleppo, Syria. Journal of King Abdulaziz University-Science, 26(2):3-18.
- Amelia, R., Akmal, W. R., & Suyono, E. A. (2023). Enhancement of astaxanthin content in mixed culture of Dunaliella sp. and Azospirillum sp. under light intensity treatment. Jurnal Ilmiah Perikanan dan Kelautan, 15(2):430-437.
- Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8):911-917.
- Borowitzka, M. A. (2018). Biology of microalgae. In I. A. Levine and J. Fleurence (Ed.), Microalgae in health and disease prevention. (pp. 23-72). Elsevier.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72:248-254.
- Chandra, R., Pradhan, S., Patel, A., & Ghosh, U. K. (2021). An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. Journal of Environmental Management, 297:113210
- Chen, M., Schliep, M., Willows., R. D., Cai, Z. L., Neilan, B. A., & Scheer, H. A. (2010). A red-shifted chlorophyll. Science, 329:1318-1319.
- Chia, S. R., Chew, K. W., Zaid, H. F. M., Chu, D., Tao, Y., & Show, P. L. (2019). Microalgal protein extraction from Chlorella vulgaris FSP-E using triphasic partitioning technique with sonication. Frontiers, 7:396.
- Dewinta, A. F., Hartono, E. S., Yusni, E., Susetya, I. E., & Siregar, R. F. (2020). The Ability of Spirulina sp. microalgae as a phytoremediation agents in liquid waste of handling fish from Cemara Market, Medan. Jurnal Ilmiah Perikanan dan Kelautan, 12(2):286–295.
- Dubois, M., Gilles, K. A., Hamilton, J. K, Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3):350-356.
- Erfianti, T., Maghfiroh, K. Q., Amelia, R., Kurnianto, D., Sadewo, B. R., Marno, S., Devi, I., Dewayanto, N., Budiman, A., & Suyono, E. A. (2023). Nitrogen sources affect the growth of local strain Euglena sp. isolated from Dieng Peatland, Central Java, Indonesia, and their potential as bio-avtur. IOP Conference Series: Earth and Environmental Science, 1151(012059):1-9.
- Erfianti, T., Wijanarka, W., & Kusumaningrum, H. P. (2021). Molecular identification of inulinolytic yeast isolated from cherry fruit (Muntingia calabura L.) based on internal transcribed spacer sequence. Journal of Physics: Conference Series, 1943(012059).
- Galví£o, R. M, Santana, T. S., Fontes, C. H. O., & Sales, E. A. (2013). Modeling of biomass production of Haematococcus pluvialis. Applied Mathemathics, 4:50-56.
- Gao, P., Guo, L., Gao, M., Zhao, Y., Jin, C., & She, Z. (2022). Regulation of carbon source metabolism in mixotrophic microalgae cultivation in response to light intensity variation. Journal of Environmental Management, 302(Part B):1-7.
- George, B., Pancha, I., Desai, C., Chokshi, K., Paliwal, C., Ghosh, T., & Mishra, S. (2014). Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – A potential strain for bio-fuel production, Bioresource Technology, 171:367-374.
- Grimm, P., Risse, J. M., Cholewa, D., Mu¨ller, J. M., Beshay, U., Friehs, K., & Flaschel, E. (2015). Applicability of Euglena gracilis for biorefineries demonstrated by the production of α-tocopherol and paramylon followed by anaerobic digestion. Journal of Biotechnology, 215:72-79.
- Grobbelaar, J. U. (2009). Upper limits of photosynthetic productivity and problems of scaling. Journal of Applied Phycology, 21(5):519-522.
- Hagiwara, S., Bolige, A., Zhang, Y., Takahashi, M., Yamagishi, A., & Goto, K. (2002). Circadian gating of photoinduction of commitment to cell-cycle transitions in relation to photoperiodic control of cell reproduction in Euglena. Photochemistry and Photobiology, 76(1):105-115.
- Hanief, S., Prasakti, L., Pradana, Y. S, Cahyono, R. B, & Budiman, A. (2020). Growth kinetic of Botryococcus braunii microalgae using Logistic and Gompertz Models. AIP Conference Proceedings, 2296(1):020065.
- He, J., Liu, C., Du, M., Zhou, X., Hu, Z., Lei, A., & Wang, J. (2021). Metabolic responses of a model green microalga Euglena gracilis to different environmental stresses. Frontiers in Bioengineering and Biotechnology, 9:662-665.
- He, M., Yan, Y., Pei, F., Wu, M., Gebreluel, T., Zou, S., & Wang, C. (2017). Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Science Reports, 7:15526.
- Iglina, T., Iglin, P., & Pashchenko, D. (2022). Industrial CO2 capture by algae: A review and recent advances. Sustainability, 14(7):3801.
- Irawan, A. P., Rahmawati, A., Fahmi, U. A., Budiman, A., Maghfiroh, K. Q., Erfianti, T., Andeska, D. P., Putri, R. A. E., Nurafifah, I., Sadewo, B., & Suyono, E. A. (2023). Studies on bioflocculant exopolysaccharides (EPS) produced by Anabaena sp. and its application as bioflocculant for low cost harvesting of Chlorella sp. Asian Journal of Agriculture and Biology, 2023(3):1-9.
- Jajesniak, P., Ali, H. E. M. O., & Wong, T. S. (2021). Carbon dioxide capture and utilization using biological systems: Opportunities and challenges. Journal of Bioprocessing & Biotechniques, 4(3):1-15.
- Janssen, M. (2002). Cultivation of microalgae: Effect of light/dark cycles on biomass yield. Wageningen University, The Netherlands: Ponsen & Looijen BV.
- Kato, S., & Nam, H. G. (2021). The cell division cycle of Euglena gracilis indicates that the level of Circadian plasticity to the external light regime changes in prolonged-stationary cultures. Plants, 10:1475.
- Khajepour, F., Hosseini, S. A., Nasrabadi, R. G., & Markou, G. (2015). Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Applied Biochemistry and Biotechnology, 176:2279-2289.
- Khoeyi, Z. A, Seyfabadi, J., & Ramezanpour, Z. (2012). Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquaculture International, 20(1):41-49.
- Kishore, G., Kadam, A. D., Kumar, U., & Arunachalam, K. (2018). Modeling Euglena sp. growth under different conditions using an artificial neural network. Journal of Applied Phycology, 30(2):955-967.
- Kitaya, Y., Azuma, H., & Kiyota, M. (2005). Effects of temperature, CO2/O2 concentrations and light intensity on cellular multiplication of microalgae, Euglena gracilis. Advances in Space Research, 35(9):1584-1588.
- Krzemińska, I., Pawlik-Skowrońska, B., Trzcińska, M., & Tys, J. (2014). Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess and Biosystems Engineering, 37(4):735-741.
- Lam, M. K., Yusoff, M. I., Uemura, Y., Lim, J. W., Khoo, C. G., Lee, K. T., & Ong, H. C. (2017). Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renewable Energy, 103:197-207.
- Lee, Y. K., & Shen, H. (2004). Basic culturing techniques. In A. Richmond (Ed.), Handbook of microalgal culture: Biotechnology and applied phycology. (pp. 40-56). Oxford: Blackwell Publishing Ltd.
- Levasseur, W., Taidi, B., Lacombe, R., Perre, P., & Pozzobon, V. (2018). Impact of seconds to minutes photoperiods on Chlorella vulgaris growth rate and chlorophyll a and b content. Algal research, 36:10-16.
- Liyanaarachchi, V. C., Nishshanka, G. K. S. H., Premaratne, R. G. M. M., Ariyadasa, T. U., Nimarshana, P. H. V., & Malik, A. (2020). Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnology Reports, 28:e0053.
- Maltsev, Y., Maltseva, K., Kulikovskiy, M., & Maltseva, S. (2021). Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology, 10(10):1060;
- Mangal, V., Donaldson, M. E., Lewis, A., Saville, B. J., & Guéguen, C. (2022). Identifying Euglena gracilis metabolic and transcriptomic adaptations in response to mercury stress. Frontiers, 10:836732.
- Mata, T. M., Melo, A. C., Simíµes, M., & Caetano, N. S. (2012). Parametric study of a brewery effluent treatment by microalgae Scenedesmus obliquus. Bioresource Technology, 107:151-158.
- Merchant, S. S., Kropat, J., Liu, B., Shaw, J. & Warakanont, J. (2012). TAG, you're it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Current Opinion in Biotechnology, 23(3):352-363.
- Minhas, A. K., Gaur, S., & Adholeya, A. (2023). Influence of light intensity and photoperiod on the pigment and, lipid production of Dunaliella tertiolecta and Nannochloropsis oculata under three different culture medium. Heliyon, e12801.
- Mondal, M., Goswami, S., Ghosh, A., Oinam, G., Tiwari, O. N, Das, P., Gayen, K., Mandal, M. K, & Halder, G. N. (2017). Production of biodiesel from microalgae through biological carbon capture: A review. Biotech, 7(2):1-21.
- Mulders, K. J. M., Lamers, P. P., Martens, D. E., & Wijffels, R. H. (2014). Phototropic pigment production with microalgae: Biological constraints and opportunities. Journal of Phycology, 50:229-242.
- Novoveská, L., Ross, M. E., Stanley, M. S., Pradelles, R., Wasiolek, V., & Sassi, J. F. (2019). Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Marine Drugs, 17(11):640.
- Nur, F., Erfianti, T., Andeska, D. P., Putri, R. A, E., Nurafifah, I., Sadewo, B. R., & Suyono, E. A. (2023). Enhancement of microalgal metabolite production through Euglena sp. local strain and glagah strain consortia. Biosaintifika, 15(1):36-47.
- Nurafifah, I., Hardianto, A., Erfianti, T., Amelia, R., Maghfiroh, K. H., Kurnianto, D., Siswanti, D. U., Sadewo, B. R., Putri, R. A. E., & Suyono, E. A. (2023). The Effect of acidic pH on growth kinetics, biomass productivity, and primary metabolite contents of Euglena sp. Makara Journal of Science, 27(2):3.
- Nzayisenga, J. C., Farge, X., Groll, S. L., & Sellstedt. (2020). Efects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnology for Biofuels and Bioproducts, 13:4. 1-8.
- Ogbonna J. C., Tanaka, H., & Tomiyamal, S. (1998). Heterotrophic cultivation of Euglena gracilis Z for efficient production of α-tocopherol. Journal of Applied Phycology, 10:67-74.
- O'Neill, E. C., Trick, M., Hill, L., Rejzek, M., Dusi, R. G., Hamilton, C. J., Zimba, P. V., Henrissat, B., & Field, R. A. (2015). The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Molecular Bio-System, 11:2808
- Phukoetphim, N., Salakkam, A., Laopaiboon, P., & Laopaiboon, L. (2017). Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations: Logistic and modified Gompertz models. Journal of Biotechnology, 243:69-75.
- Pulz, O. (2001). Photobioreactors: Production systems for phototrophic microorganisms. Applied Microbiology and Biotechnology, 57(3):287–293.
- Richmond, A. (2004). Handbook of microalgal culture. Oxford: Blackwell Science Ltd.
- Richter, P. R., Strauch, S. M., Ntefidou, M., Schuster, M., Daiker, V., Nasir, A., Haag, F. W. M., & Lebert, M. (2014). Influence of different light-dark cycles on motility and photosynthesis of Euglena gracilis in closed bioreactors. Astrobiology, 14(10):848-858.
- Ruangsomboon, S. (2012). Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresource Technology, 109:261-265.
- Scaife, M. A., Nguyen, G. T. D. T., Rico, J., Lambert, D., Helliwell, K. E., & Smith, A. G. (2015). Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. The Plant Journal, 82:532-546.
- Seckbach, J., & Libby, W. F. (1970). Vegetative life on Venus ? Or investigations with algae which grow under pure CO2 in hot acid media and at elevated pressures. Space Life Sci, 2(2):121-143.
- Seyfabadi, J., Ramezanpour, Z., & Khoeyi, Z. A. (2011). Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. Journal of Applied Phycology, 23(4):721-726.
- Shi, T. Q., Wang, L. R., Zhang, Z. X., Sun, X. M., & Huang, H. (2020). Stresses as first-line tools for enhancing lipid and carotenoid production in microalgae. Frontiers, 8:610.
- Solovchenko, A. E. (2015). Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynthesis Research, 125:437-449.
- Subramanian, S., Barry, A. N., Pieris, S., & Sayre, R. T. (2013). Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: Implications for biomass and biofuel production. Biotechnology for Biofuels, 6(1):150.
- Suzuki, K. (2017). Large-scale cultivation of Euglena. In S. D. Schwartzbach and S. Shigeoka (Ed.), Euglena: Biochemistry, cell and molecular biology. (pp. 285–293). Cham: Springer.
- Suzuki, K., Mitra, S., Iwata, O., Ishikawa, T., Kato, S., & Yamada, K. (2015). Selection and characterization of Euglena anabaena var. minor as a new candidate Euglena species for industrial application. Bioscience, Biotechnology & Biochemistry, 79(10):1730-1736.
- Tamaki, S., Tanno, Y., Kato, S., Ozasa, K., Wakazaki, M., Sato, M., Toyooka, K., Maoka, T., Ishikawa, T., & Maeda, M., (2020). Carotenoid accumulation in the eyespot apparatus required for phototaxis is independent of chloroplast development in Euglena gracilis. Plant Science, 298:110564.
- Thrane, J. E., Kyle, M., Striebel, M., Haande, S., Grung, M., Rohrlack, T., & Anderen, T. (2015). Spectrophotometric analysis of pigments: A critical assessment of a high-throughput method for analysis of algal pigment mixtures by spectral deconvolution. PLoS ONE, 10(9):e0137645.
- Vecchi, V., Barera, S., Bassi, R., & Dall'osto, L. (2020). Potential and challenges of improving photosynthesis in algae. Plants, 9(1):67.
- Vélez-Landa, L., Hernández-De, L. H. R., Pérez-Luna, Y. D. C, Velázquez-Trujillo, S., Moreira-Acosta, J., Berrones-Hernández, R., & Sánchez-Roque, Y. (2021). Influence of light intensity and photoperiod on the photoautotrophic growth and lipid content of the microalgae Verrucodesmus verrucosus in a photobioreactor. Sustainability, 13(12):6606.
- Wahidin, S., Idris, A., & Shaleh, S. R. (2013). The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresource Technology, 129:7-11.
- Wang, Y., Seppa¨nen-Laakso, T., Rischer, H., & Wiebe, M. G. (2018). Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS ONE, 13(4):e0195329.
- Yoshioka, K., Suzuki, K., & Osanai, T. (2020). Effect of pH on metabolite excretion and cell morphology of Euglena gracilis under dark, anaerobic conditions. Algal Research, 51:102084.
- Zulkarnain, M. I., Rahayu, H. T., Herida, A. P, Erfianti, T., Kusumaningrum, H. P., Zainuri, M., Endrawati, H., Widowati, I., Haryanti, W. D. U., & Mahendrajaya, R. T. (2021). Improvement of the potency of microalgae Nannochloropsis and Chaetoceros through antioxidant analysis and optimization of DNA isolation. Journal of Physics: Conference Series, 1943(012080).
References
Al-Ashra, M S., Abiad, M., & Allahem, A. (2014). Morphological and molecular taxonomical study of Euglena viridis ehren and Euglena gracilis klebs growing in Aleppo, Syria. Journal of King Abdulaziz University-Science, 26(2):3-18.
Amelia, R., Akmal, W. R., & Suyono, E. A. (2023). Enhancement of astaxanthin content in mixed culture of Dunaliella sp. and Azospirillum sp. under light intensity treatment. Jurnal Ilmiah Perikanan dan Kelautan, 15(2):430-437.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8):911-917.
Borowitzka, M. A. (2018). Biology of microalgae. In I. A. Levine and J. Fleurence (Ed.), Microalgae in health and disease prevention. (pp. 23-72). Elsevier.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72:248-254.
Chandra, R., Pradhan, S., Patel, A., & Ghosh, U. K. (2021). An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. Journal of Environmental Management, 297:113210
Chen, M., Schliep, M., Willows., R. D., Cai, Z. L., Neilan, B. A., & Scheer, H. A. (2010). A red-shifted chlorophyll. Science, 329:1318-1319.
Chia, S. R., Chew, K. W., Zaid, H. F. M., Chu, D., Tao, Y., & Show, P. L. (2019). Microalgal protein extraction from Chlorella vulgaris FSP-E using triphasic partitioning technique with sonication. Frontiers, 7:396.
Dewinta, A. F., Hartono, E. S., Yusni, E., Susetya, I. E., & Siregar, R. F. (2020). The Ability of Spirulina sp. microalgae as a phytoremediation agents in liquid waste of handling fish from Cemara Market, Medan. Jurnal Ilmiah Perikanan dan Kelautan, 12(2):286–295.
Dubois, M., Gilles, K. A., Hamilton, J. K, Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3):350-356.
Erfianti, T., Maghfiroh, K. Q., Amelia, R., Kurnianto, D., Sadewo, B. R., Marno, S., Devi, I., Dewayanto, N., Budiman, A., & Suyono, E. A. (2023). Nitrogen sources affect the growth of local strain Euglena sp. isolated from Dieng Peatland, Central Java, Indonesia, and their potential as bio-avtur. IOP Conference Series: Earth and Environmental Science, 1151(012059):1-9.
Erfianti, T., Wijanarka, W., & Kusumaningrum, H. P. (2021). Molecular identification of inulinolytic yeast isolated from cherry fruit (Muntingia calabura L.) based on internal transcribed spacer sequence. Journal of Physics: Conference Series, 1943(012059).
Galví£o, R. M, Santana, T. S., Fontes, C. H. O., & Sales, E. A. (2013). Modeling of biomass production of Haematococcus pluvialis. Applied Mathemathics, 4:50-56.
Gao, P., Guo, L., Gao, M., Zhao, Y., Jin, C., & She, Z. (2022). Regulation of carbon source metabolism in mixotrophic microalgae cultivation in response to light intensity variation. Journal of Environmental Management, 302(Part B):1-7.
George, B., Pancha, I., Desai, C., Chokshi, K., Paliwal, C., Ghosh, T., & Mishra, S. (2014). Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – A potential strain for bio-fuel production, Bioresource Technology, 171:367-374.
Grimm, P., Risse, J. M., Cholewa, D., Mu¨ller, J. M., Beshay, U., Friehs, K., & Flaschel, E. (2015). Applicability of Euglena gracilis for biorefineries demonstrated by the production of α-tocopherol and paramylon followed by anaerobic digestion. Journal of Biotechnology, 215:72-79.
Grobbelaar, J. U. (2009). Upper limits of photosynthetic productivity and problems of scaling. Journal of Applied Phycology, 21(5):519-522.
Hagiwara, S., Bolige, A., Zhang, Y., Takahashi, M., Yamagishi, A., & Goto, K. (2002). Circadian gating of photoinduction of commitment to cell-cycle transitions in relation to photoperiodic control of cell reproduction in Euglena. Photochemistry and Photobiology, 76(1):105-115.
Hanief, S., Prasakti, L., Pradana, Y. S, Cahyono, R. B, & Budiman, A. (2020). Growth kinetic of Botryococcus braunii microalgae using Logistic and Gompertz Models. AIP Conference Proceedings, 2296(1):020065.
He, J., Liu, C., Du, M., Zhou, X., Hu, Z., Lei, A., & Wang, J. (2021). Metabolic responses of a model green microalga Euglena gracilis to different environmental stresses. Frontiers in Bioengineering and Biotechnology, 9:662-665.
He, M., Yan, Y., Pei, F., Wu, M., Gebreluel, T., Zou, S., & Wang, C. (2017). Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Science Reports, 7:15526.
Iglina, T., Iglin, P., & Pashchenko, D. (2022). Industrial CO2 capture by algae: A review and recent advances. Sustainability, 14(7):3801.
Irawan, A. P., Rahmawati, A., Fahmi, U. A., Budiman, A., Maghfiroh, K. Q., Erfianti, T., Andeska, D. P., Putri, R. A. E., Nurafifah, I., Sadewo, B., & Suyono, E. A. (2023). Studies on bioflocculant exopolysaccharides (EPS) produced by Anabaena sp. and its application as bioflocculant for low cost harvesting of Chlorella sp. Asian Journal of Agriculture and Biology, 2023(3):1-9.
Jajesniak, P., Ali, H. E. M. O., & Wong, T. S. (2021). Carbon dioxide capture and utilization using biological systems: Opportunities and challenges. Journal of Bioprocessing & Biotechniques, 4(3):1-15.
Janssen, M. (2002). Cultivation of microalgae: Effect of light/dark cycles on biomass yield. Wageningen University, The Netherlands: Ponsen & Looijen BV.
Kato, S., & Nam, H. G. (2021). The cell division cycle of Euglena gracilis indicates that the level of Circadian plasticity to the external light regime changes in prolonged-stationary cultures. Plants, 10:1475.
Khajepour, F., Hosseini, S. A., Nasrabadi, R. G., & Markou, G. (2015). Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Applied Biochemistry and Biotechnology, 176:2279-2289.
Khoeyi, Z. A, Seyfabadi, J., & Ramezanpour, Z. (2012). Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquaculture International, 20(1):41-49.
Kishore, G., Kadam, A. D., Kumar, U., & Arunachalam, K. (2018). Modeling Euglena sp. growth under different conditions using an artificial neural network. Journal of Applied Phycology, 30(2):955-967.
Kitaya, Y., Azuma, H., & Kiyota, M. (2005). Effects of temperature, CO2/O2 concentrations and light intensity on cellular multiplication of microalgae, Euglena gracilis. Advances in Space Research, 35(9):1584-1588.
Krzemińska, I., Pawlik-Skowrońska, B., Trzcińska, M., & Tys, J. (2014). Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess and Biosystems Engineering, 37(4):735-741.
Lam, M. K., Yusoff, M. I., Uemura, Y., Lim, J. W., Khoo, C. G., Lee, K. T., & Ong, H. C. (2017). Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renewable Energy, 103:197-207.
Lee, Y. K., & Shen, H. (2004). Basic culturing techniques. In A. Richmond (Ed.), Handbook of microalgal culture: Biotechnology and applied phycology. (pp. 40-56). Oxford: Blackwell Publishing Ltd.
Levasseur, W., Taidi, B., Lacombe, R., Perre, P., & Pozzobon, V. (2018). Impact of seconds to minutes photoperiods on Chlorella vulgaris growth rate and chlorophyll a and b content. Algal research, 36:10-16.
Liyanaarachchi, V. C., Nishshanka, G. K. S. H., Premaratne, R. G. M. M., Ariyadasa, T. U., Nimarshana, P. H. V., & Malik, A. (2020). Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnology Reports, 28:e0053.
Maltsev, Y., Maltseva, K., Kulikovskiy, M., & Maltseva, S. (2021). Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology, 10(10):1060;
Mangal, V., Donaldson, M. E., Lewis, A., Saville, B. J., & Guéguen, C. (2022). Identifying Euglena gracilis metabolic and transcriptomic adaptations in response to mercury stress. Frontiers, 10:836732.
Mata, T. M., Melo, A. C., Simíµes, M., & Caetano, N. S. (2012). Parametric study of a brewery effluent treatment by microalgae Scenedesmus obliquus. Bioresource Technology, 107:151-158.
Merchant, S. S., Kropat, J., Liu, B., Shaw, J. & Warakanont, J. (2012). TAG, you're it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Current Opinion in Biotechnology, 23(3):352-363.
Minhas, A. K., Gaur, S., & Adholeya, A. (2023). Influence of light intensity and photoperiod on the pigment and, lipid production of Dunaliella tertiolecta and Nannochloropsis oculata under three different culture medium. Heliyon, e12801.
Mondal, M., Goswami, S., Ghosh, A., Oinam, G., Tiwari, O. N, Das, P., Gayen, K., Mandal, M. K, & Halder, G. N. (2017). Production of biodiesel from microalgae through biological carbon capture: A review. Biotech, 7(2):1-21.
Mulders, K. J. M., Lamers, P. P., Martens, D. E., & Wijffels, R. H. (2014). Phototropic pigment production with microalgae: Biological constraints and opportunities. Journal of Phycology, 50:229-242.
Novoveská, L., Ross, M. E., Stanley, M. S., Pradelles, R., Wasiolek, V., & Sassi, J. F. (2019). Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Marine Drugs, 17(11):640.
Nur, F., Erfianti, T., Andeska, D. P., Putri, R. A, E., Nurafifah, I., Sadewo, B. R., & Suyono, E. A. (2023). Enhancement of microalgal metabolite production through Euglena sp. local strain and glagah strain consortia. Biosaintifika, 15(1):36-47.
Nurafifah, I., Hardianto, A., Erfianti, T., Amelia, R., Maghfiroh, K. H., Kurnianto, D., Siswanti, D. U., Sadewo, B. R., Putri, R. A. E., & Suyono, E. A. (2023). The Effect of acidic pH on growth kinetics, biomass productivity, and primary metabolite contents of Euglena sp. Makara Journal of Science, 27(2):3.
Nzayisenga, J. C., Farge, X., Groll, S. L., & Sellstedt. (2020). Efects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnology for Biofuels and Bioproducts, 13:4. 1-8.
Ogbonna J. C., Tanaka, H., & Tomiyamal, S. (1998). Heterotrophic cultivation of Euglena gracilis Z for efficient production of α-tocopherol. Journal of Applied Phycology, 10:67-74.
O'Neill, E. C., Trick, M., Hill, L., Rejzek, M., Dusi, R. G., Hamilton, C. J., Zimba, P. V., Henrissat, B., & Field, R. A. (2015). The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Molecular Bio-System, 11:2808
Phukoetphim, N., Salakkam, A., Laopaiboon, P., & Laopaiboon, L. (2017). Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations: Logistic and modified Gompertz models. Journal of Biotechnology, 243:69-75.
Pulz, O. (2001). Photobioreactors: Production systems for phototrophic microorganisms. Applied Microbiology and Biotechnology, 57(3):287–293.
Richmond, A. (2004). Handbook of microalgal culture. Oxford: Blackwell Science Ltd.
Richter, P. R., Strauch, S. M., Ntefidou, M., Schuster, M., Daiker, V., Nasir, A., Haag, F. W. M., & Lebert, M. (2014). Influence of different light-dark cycles on motility and photosynthesis of Euglena gracilis in closed bioreactors. Astrobiology, 14(10):848-858.
Ruangsomboon, S. (2012). Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresource Technology, 109:261-265.
Scaife, M. A., Nguyen, G. T. D. T., Rico, J., Lambert, D., Helliwell, K. E., & Smith, A. G. (2015). Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. The Plant Journal, 82:532-546.
Seckbach, J., & Libby, W. F. (1970). Vegetative life on Venus ? Or investigations with algae which grow under pure CO2 in hot acid media and at elevated pressures. Space Life Sci, 2(2):121-143.
Seyfabadi, J., Ramezanpour, Z., & Khoeyi, Z. A. (2011). Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. Journal of Applied Phycology, 23(4):721-726.
Shi, T. Q., Wang, L. R., Zhang, Z. X., Sun, X. M., & Huang, H. (2020). Stresses as first-line tools for enhancing lipid and carotenoid production in microalgae. Frontiers, 8:610.
Solovchenko, A. E. (2015). Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynthesis Research, 125:437-449.
Subramanian, S., Barry, A. N., Pieris, S., & Sayre, R. T. (2013). Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: Implications for biomass and biofuel production. Biotechnology for Biofuels, 6(1):150.
Suzuki, K. (2017). Large-scale cultivation of Euglena. In S. D. Schwartzbach and S. Shigeoka (Ed.), Euglena: Biochemistry, cell and molecular biology. (pp. 285–293). Cham: Springer.
Suzuki, K., Mitra, S., Iwata, O., Ishikawa, T., Kato, S., & Yamada, K. (2015). Selection and characterization of Euglena anabaena var. minor as a new candidate Euglena species for industrial application. Bioscience, Biotechnology & Biochemistry, 79(10):1730-1736.
Tamaki, S., Tanno, Y., Kato, S., Ozasa, K., Wakazaki, M., Sato, M., Toyooka, K., Maoka, T., Ishikawa, T., & Maeda, M., (2020). Carotenoid accumulation in the eyespot apparatus required for phototaxis is independent of chloroplast development in Euglena gracilis. Plant Science, 298:110564.
Thrane, J. E., Kyle, M., Striebel, M., Haande, S., Grung, M., Rohrlack, T., & Anderen, T. (2015). Spectrophotometric analysis of pigments: A critical assessment of a high-throughput method for analysis of algal pigment mixtures by spectral deconvolution. PLoS ONE, 10(9):e0137645.
Vecchi, V., Barera, S., Bassi, R., & Dall'osto, L. (2020). Potential and challenges of improving photosynthesis in algae. Plants, 9(1):67.
Vélez-Landa, L., Hernández-De, L. H. R., Pérez-Luna, Y. D. C, Velázquez-Trujillo, S., Moreira-Acosta, J., Berrones-Hernández, R., & Sánchez-Roque, Y. (2021). Influence of light intensity and photoperiod on the photoautotrophic growth and lipid content of the microalgae Verrucodesmus verrucosus in a photobioreactor. Sustainability, 13(12):6606.
Wahidin, S., Idris, A., & Shaleh, S. R. (2013). The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresource Technology, 129:7-11.
Wang, Y., Seppa¨nen-Laakso, T., Rischer, H., & Wiebe, M. G. (2018). Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS ONE, 13(4):e0195329.
Yoshioka, K., Suzuki, K., & Osanai, T. (2020). Effect of pH on metabolite excretion and cell morphology of Euglena gracilis under dark, anaerobic conditions. Algal Research, 51:102084.
Zulkarnain, M. I., Rahayu, H. T., Herida, A. P, Erfianti, T., Kusumaningrum, H. P., Zainuri, M., Endrawati, H., Widowati, I., Haryanti, W. D. U., & Mahendrajaya, R. T. (2021). Improvement of the potency of microalgae Nannochloropsis and Chaetoceros through antioxidant analysis and optimization of DNA isolation. Journal of Physics: Conference Series, 1943(012080).