Date Log
Copyright (c) 2023 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
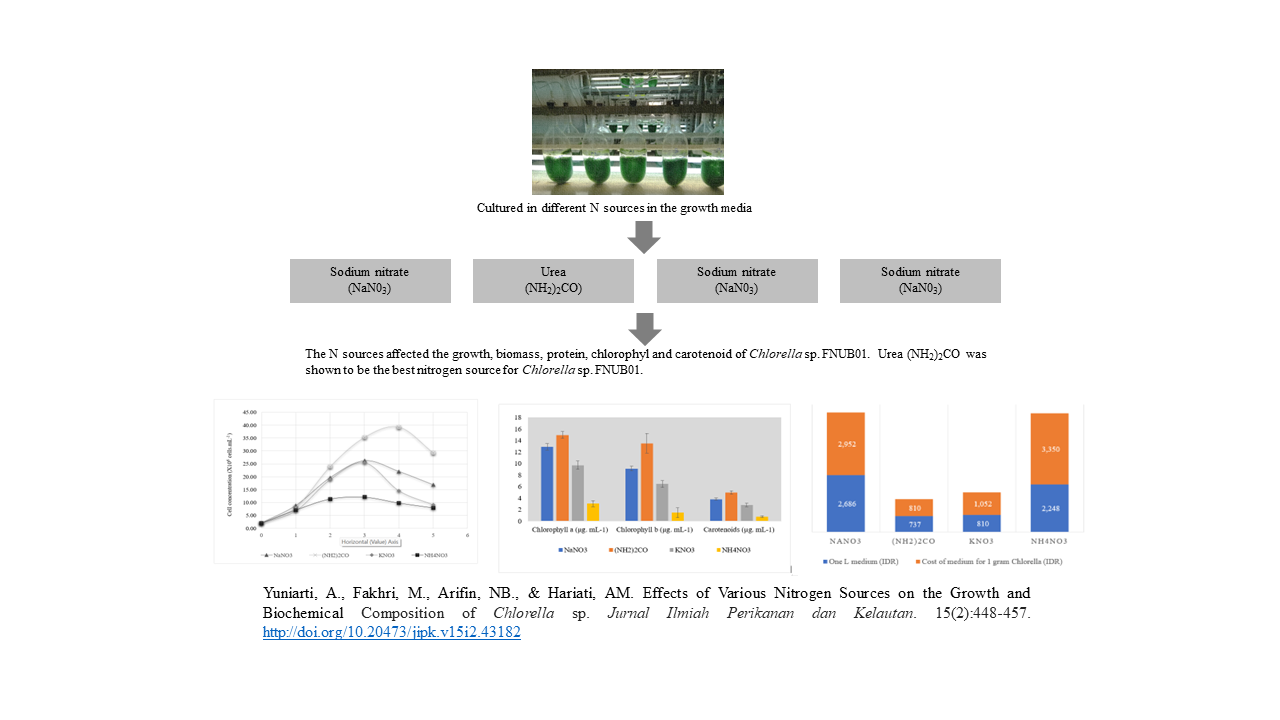
Effects of Various Nitrogen Sources on the Growth and Biochemical Composition of Chlorella sp.
Corresponding Author(s) : Ating Yuniarti
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 15 No. 2 (2023): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Highlight Research
- Each species of microalga has a preferable nitrogen source for their optimal growth.
- The nitrogen sources in the grown media affected the growth rate and biochemical composition of Chlorella FNUB01.
- (NH2)2CO (urea) was found to be the best alternative nitrogen source for Chlorella FNUB01.
- For producing 1 g of Chlorella FNUB01, the use of urea reduced the cost of medium by 72.6%.
Abstract
Chlorella sp. is a potential microalgae species to be produced commercially for feed, growth accelerator, and immuno-modulator in fish and shrimp culture. This study aimed to evaluate the various nitrogen sources on the growth, biomass production, and biochemical composition of Chlorella sp. FNUB01. The nitrogen sources used in this study were urea (NH2)2CO, potassium nitrate (KNO3), and ammonium nitrate (NH4NO3). Sodium nitrate (NaNO3) was used as a control as it is a part of the commercial medium BG-11. Generally, the sources of nitrogen in the media affected the growth and chemical composition of Chlorella sp. FNUB01. This green microalga grew better in the urea-containing medium which accounted for 1.5 times the concentration of that cultured in BG-11 (40 x106 cells. mL-1). Meanwhile, this microalgae species experienced the lowest growth when cultured in NH4NO3-containing medium. The biomass productivity of Chlorella sp. FNUB01 cultured in urea (0.93 g.L-1) was comparable to those grown with NaNO3 as the N source. A similar pattern was recorded for protein, chlorophyll, and carotenoid content as these biochemical contents were affected by N availability in the medium. Urea was an alternative low-cost N source for the culture of Chlorella sp. FNUB01. Replacement of NaNO3 with urea could reduce the cost of the medium by 72.6%.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Ahmad, M. T., Shariff, M., Yusoff, F. Md., Goh, Y. M., & Banerjee, S. (2018). Applications of microalga Chlorella vulgaris in aquaculture. Reviews in Aquaculture, 12(5):328–346.
- Amin, N. F., Khalafallah, M. A., Ali, M. A., Abou-Sdera, S. A., & Matter, I. A. (2013). Effect of some nitrogen sources on growth and lipid of microalgae Chlorella sp. for biodiesel production. Journal of Applied Sciences Research, 9(8):4845-4855.
- Bezerra, R. P., Matsudo, M. C., Sato, S., Converti, A., & de Carvalho, J. C. M. (2013). Fed-batch cultivation of Arthrospira platensis using carbon dioxide from alcoholic fermentation and urea as carbon and nitrogen sources. Bioenergy Research, 6(3):1118-1125.
- Borowitzka, M., & Borowitzka, L. (1988). Limits to growth and carotenogenesis in laboratory and large-scale outdoor cultures of Dunaliella salina. In T. Stadler, J. Mollion, M. C. Verdus, Y. Karamanos, H. Morvan, & D. Christiaen (Ed.), Algal biotechnology. (pp. 371-381). Canada: Elsevier Applied Science.
- Cabello, P., Luque-Almagro, V. M., Roldán, M. D., & Moreno-Vivián, C. (2019). Nitrogen cycle. Encyclopedia of Microbiology, 3:301-310.
- Caspi, R., Billington, R., Ferrer, L., Foerster, H., Fulcher, C. A., Keseler, I. M., Kothari, A., Krummenacker, M., Latendresse, M., Mueller, L. A., Ong, Q., Paley, S., Subhraveti, P., Weaver, D. S., & Karp, P. D. (2016). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Research, 44(D1):D471-D480.
- Chacón-Lee, T. L., & González-Mariño, G. E. (2010). Microalgae for "healthy” foods-possibilities and challenges. Comprehensive Reviews in Food Science and Food Safety, 9(6):655-675.
- Cheah, W. Y., Show, P. L., Chang, J. S., Ling, T. C., & Juan, J. C. (2015). Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresource Technology, 184:190-201.
- Chen, C. Y., Lee, P. J., Tan, C. H., Lo, Y. C., Huang, C. C., Show, P. L., Lin, C. H., & Chang, J. S. (2015). Improving protein production of indigenous microalga Chlorella vulgaris FSP-E by photobioreactor design and cultivation strategies. Biotechnology Journal, 10(6):905-914.
- Chowdury, K. H., Nahar, N., & Deb, U. K. (2020). The Growth factors involved in microalgae cultivation for biofuel production: A review. Computational Water, Energy, and Environmental Engineering, 09(04):185-215.
- Coulombier, N., Nicolau, E., Déan, L. L., Barthelemy, V., Schreiber, N., Brun, P., Lebouvier, N., & Jauffrais, T. (2020). Effects of nitrogen availability on the antioxidant activity and carotenoid content of the microalgae Nephroselmis sp. Marine Drugs, 18(453):1-22.
- Fakhri, M., Antika, P. W., Ekawati, A. W., & Arifin, N. B. (2020). Growth, pigment content, and protein of Spirulina platensis cultured in Ca(NO3)2 with different doses . Journal of Aquaculture and Fish Health, 9(1):38-47.
- Fakhri, M., Riyani, E., Ekawati, A. W., Arifin, N. B., Yuniarti, A., Widyawati, Y., Saputra, I. K., Samuel, P. D., Arif, M. Z., & Hariati, A. M. (2021). Biomass, pigment production, and nutrient uptake of Chlorella sp. Under different photoperiods. Biodiversitas, 22(12):5344-5349.
- Fathi, M., Meshkini, S., & Nadiri, R. (2013). The effect of extracted salt from Urmia Lake on the growth, βeta- carotene and chlorophyll a content of halophilic alga Chlorella sp. Mojtaba. Turkish Journal of Fisheries and Aquatic Sciences, 13(2):233-240.
- Göksan, T., Ak, I., & Kiliç, C. (2011). Growth characteristics of the alga Haematococcus pluvialis flotow as affected by nitrogen source, vitamin, light and aeration. Turkish Journal of Fisheries and Aquatic Sciences, 11(3):377-383.
- Keleştemur, G., & Çoban, O. (2016). Effects of the β-carotene on the growth performance and skin pigmentation of rainbow trout (Oncorhynchus mykiss, W. 1792). Journal of Fisheries & Livestock Production, 4(1):3-6.
- Kim, S., Lee, Y., & Hwang, S. J. (2013). Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. International Biodeterioration and Biodegradation, 85:511-516.
- Lai, Y. C., Karam, A. L., Sederoff, H. W., Ducoste, J. J., & de los Reyes, F. L. (2019). Relating nitrogen concentration and light intensity to the growth and lipid accumulation of Dunaliella viridis in a photobioreactor. Journal of Applied Phycology, 31(6):3397-3409.
- Li, X., Li, W., Zhai, J., Wei, H., & Wang, Q. (2019). Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresource Technology, 273:368-376.
- Liefer, J. D., Garg, A., Campbell, D. A., Irwin, A. J., & Finkel, Z. V. (2018). Nitrogen starvation induces distinct photosynthetic responses and recovery dynamics in diatoms and prasinophytes. PLoS ONE, 13(4):1-24.
- Lin, Q., & Lin, J. (2011). Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresource Technology, 102(2):1615-1621.
- Lourenço, S. O., Barbarino, E., Mancini-Filho, J., Schinke, K. P., & Aidar, E. (2002). Effects of different nitrogen sources on the growth and biochemical profile of 10 marine microalgae in batch culture: An evaluation for aquaculture. Phycologia, 41(2):158-168.
- Lv, J. M., Cheng, L. H., Xu, X. H., Zhang, L., & Chen, H. L. (2010). Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresource Technology, 101(17):6797-6804.
- MakareviÄienÄ—, V., AndruleviÄiÅ«tÄ—, V., SkorupskaitÄ—, V., & KasperoviÄienÄ—, J. (2011). Cultivation of microalgae Chlorella sp. and Scenedesmus sp. as a potentional biofuel feedstock. Environmental Research, Engineering and Management, 3(57):21-27.
- Matsudo, M. C., Bezerra, R. P., Sato, S., Perego, P., Converti, A., & Carvalho, J. C. M. (2009). Repeated fed-batch cultivation of Arthrospira (Spirulina) platensis using urea as nitrogen source. Biochemical Engineering Journal, 43(1):52-57.
- Pinton, R., Tomasi, N., & Zanin, L. (2016). Molecular and physiological interactions of urea and nitrate uptake in plants. Plant Signaling & Behavior, 11(1):e1076603.
- Podevin, M., De Francisci, D., Holdt, S. L., & Angelidaki, I. (2015). Effect of nitrogen source and acclimatization on specific growth rates of microalgae determined by a high-throughput in vivo microplate autofluorescence method. Journal of Applied Phycology, 27(4):1415-1423.
- Qiu, R., Gao, S., Lopez, P. A., & Ogden, K. L. (2017). Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Research, 28:192-199.
- Ramanna, L., Guldhe, A., Rawat, I., & Bux, F. (2014). The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresource Technology, 168:127-135.
- Raven, J. A., & Giordano, M. (2016). Combined nitrogen. In M. Borowitzka, J. Beardall, & J. Raven (Ed.), The physiology of microalgae. (pp. 143-154). Switzerland: Springer International Publishing.
- Ribeiro, D. M., Roncaratti, L. F., Possa, G. C., Garcia, L. C., Cançado, L. J., Williams, T. C. R., & Brasil, B. dos S. A. F. (2020). A low-cost approach for Chlorella sorokiniana production through combined use of urea, ammonia and nitrate based fertilizers. Bioresource Technology Reports, 9:100354.
- Rodrigues, M. S., Ferreira, L. S., Converti, A., Sato, S., & de Carvalho, J. C. M. (2011). Influence of ammonium sulphate feeding time on fed-batch Arthrospira (Spirulina) platensis cultivation and biomass composition with and without pH control. Bioresource Technology, 102(11):6587-6592.
- Safafar, H., Ní¸rregaard, P. U., Ljubic, A., Mí¸ller, P., Holdt, S. L., & Jacobsen, C. (2016). Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. Journal of Marine Science and Engineering, 4(48):1-15.
- Scherholz, M. L., & Curtis, W. R. (2015). Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnology, 13(39):1-6.
- Singh, S. P., & Singh, P. (2014). Effect of CO2 concentration on algal growth: A review. Renewable and Sustainable Energy Reviews, 38:172-179.
- Takaichi, S. (2011). Carotenoids in algae: Distributions, biosyntheses and functions. Marine Drugs, 9(6):1101-1118.
- Wang, J., Sommerfeld, M. R., Lu, C., & Hu, Q. (2013). Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae, 28(2):193-202.
- Xin, L., Hong-ying, H., Ke, G., & Ying-xue, S. (2010). Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresource Technology, 101(14):5494-5500.
- Xu, N., Zhang, X., Fan, X., Han, L., & Zeng, C. (2001). Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). Journal of Applied Phycology, 13:463-469.
- Xu, Y., Ibrahim, I. M., & Harvey, P. J. (2016). The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiology and Biochemistry, 106:305-315.
- Yaakob, Z., Ali, E., Zainal, A., Mohamad, M., & Takriff, M. S. (2014). An overview: biomolecules from microalgae for animal feed and aquaculture. Journal of Biological Research – Thessaloniki, 21(6):1-10.
- Young, E. B., & Beardall, J. (2003). Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. Journal of Phycology, 39(5):897-905.
- Yuan, C., Xu, K., Sun, J., Hu, G. R., & Li, F. L. (2018). Ammonium, nitrate, and urea play different roles for lipid accumulation in the nervonic acid”producing microalgae Mychonastes afer HSO-3-1. Journal of Applied Phycology, 30(2):793-801.
References
Ahmad, M. T., Shariff, M., Yusoff, F. Md., Goh, Y. M., & Banerjee, S. (2018). Applications of microalga Chlorella vulgaris in aquaculture. Reviews in Aquaculture, 12(5):328–346.
Amin, N. F., Khalafallah, M. A., Ali, M. A., Abou-Sdera, S. A., & Matter, I. A. (2013). Effect of some nitrogen sources on growth and lipid of microalgae Chlorella sp. for biodiesel production. Journal of Applied Sciences Research, 9(8):4845-4855.
Bezerra, R. P., Matsudo, M. C., Sato, S., Converti, A., & de Carvalho, J. C. M. (2013). Fed-batch cultivation of Arthrospira platensis using carbon dioxide from alcoholic fermentation and urea as carbon and nitrogen sources. Bioenergy Research, 6(3):1118-1125.
Borowitzka, M., & Borowitzka, L. (1988). Limits to growth and carotenogenesis in laboratory and large-scale outdoor cultures of Dunaliella salina. In T. Stadler, J. Mollion, M. C. Verdus, Y. Karamanos, H. Morvan, & D. Christiaen (Ed.), Algal biotechnology. (pp. 371-381). Canada: Elsevier Applied Science.
Cabello, P., Luque-Almagro, V. M., Roldán, M. D., & Moreno-Vivián, C. (2019). Nitrogen cycle. Encyclopedia of Microbiology, 3:301-310.
Caspi, R., Billington, R., Ferrer, L., Foerster, H., Fulcher, C. A., Keseler, I. M., Kothari, A., Krummenacker, M., Latendresse, M., Mueller, L. A., Ong, Q., Paley, S., Subhraveti, P., Weaver, D. S., & Karp, P. D. (2016). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Research, 44(D1):D471-D480.
Chacón-Lee, T. L., & González-Mariño, G. E. (2010). Microalgae for "healthy” foods-possibilities and challenges. Comprehensive Reviews in Food Science and Food Safety, 9(6):655-675.
Cheah, W. Y., Show, P. L., Chang, J. S., Ling, T. C., & Juan, J. C. (2015). Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresource Technology, 184:190-201.
Chen, C. Y., Lee, P. J., Tan, C. H., Lo, Y. C., Huang, C. C., Show, P. L., Lin, C. H., & Chang, J. S. (2015). Improving protein production of indigenous microalga Chlorella vulgaris FSP-E by photobioreactor design and cultivation strategies. Biotechnology Journal, 10(6):905-914.
Chowdury, K. H., Nahar, N., & Deb, U. K. (2020). The Growth factors involved in microalgae cultivation for biofuel production: A review. Computational Water, Energy, and Environmental Engineering, 09(04):185-215.
Coulombier, N., Nicolau, E., Déan, L. L., Barthelemy, V., Schreiber, N., Brun, P., Lebouvier, N., & Jauffrais, T. (2020). Effects of nitrogen availability on the antioxidant activity and carotenoid content of the microalgae Nephroselmis sp. Marine Drugs, 18(453):1-22.
Fakhri, M., Antika, P. W., Ekawati, A. W., & Arifin, N. B. (2020). Growth, pigment content, and protein of Spirulina platensis cultured in Ca(NO3)2 with different doses . Journal of Aquaculture and Fish Health, 9(1):38-47.
Fakhri, M., Riyani, E., Ekawati, A. W., Arifin, N. B., Yuniarti, A., Widyawati, Y., Saputra, I. K., Samuel, P. D., Arif, M. Z., & Hariati, A. M. (2021). Biomass, pigment production, and nutrient uptake of Chlorella sp. Under different photoperiods. Biodiversitas, 22(12):5344-5349.
Fathi, M., Meshkini, S., & Nadiri, R. (2013). The effect of extracted salt from Urmia Lake on the growth, βeta- carotene and chlorophyll a content of halophilic alga Chlorella sp. Mojtaba. Turkish Journal of Fisheries and Aquatic Sciences, 13(2):233-240.
Göksan, T., Ak, I., & Kiliç, C. (2011). Growth characteristics of the alga Haematococcus pluvialis flotow as affected by nitrogen source, vitamin, light and aeration. Turkish Journal of Fisheries and Aquatic Sciences, 11(3):377-383.
Keleştemur, G., & Çoban, O. (2016). Effects of the β-carotene on the growth performance and skin pigmentation of rainbow trout (Oncorhynchus mykiss, W. 1792). Journal of Fisheries & Livestock Production, 4(1):3-6.
Kim, S., Lee, Y., & Hwang, S. J. (2013). Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. International Biodeterioration and Biodegradation, 85:511-516.
Lai, Y. C., Karam, A. L., Sederoff, H. W., Ducoste, J. J., & de los Reyes, F. L. (2019). Relating nitrogen concentration and light intensity to the growth and lipid accumulation of Dunaliella viridis in a photobioreactor. Journal of Applied Phycology, 31(6):3397-3409.
Li, X., Li, W., Zhai, J., Wei, H., & Wang, Q. (2019). Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresource Technology, 273:368-376.
Liefer, J. D., Garg, A., Campbell, D. A., Irwin, A. J., & Finkel, Z. V. (2018). Nitrogen starvation induces distinct photosynthetic responses and recovery dynamics in diatoms and prasinophytes. PLoS ONE, 13(4):1-24.
Lin, Q., & Lin, J. (2011). Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresource Technology, 102(2):1615-1621.
Lourenço, S. O., Barbarino, E., Mancini-Filho, J., Schinke, K. P., & Aidar, E. (2002). Effects of different nitrogen sources on the growth and biochemical profile of 10 marine microalgae in batch culture: An evaluation for aquaculture. Phycologia, 41(2):158-168.
Lv, J. M., Cheng, L. H., Xu, X. H., Zhang, L., & Chen, H. L. (2010). Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresource Technology, 101(17):6797-6804.
MakareviÄienÄ—, V., AndruleviÄiÅ«tÄ—, V., SkorupskaitÄ—, V., & KasperoviÄienÄ—, J. (2011). Cultivation of microalgae Chlorella sp. and Scenedesmus sp. as a potentional biofuel feedstock. Environmental Research, Engineering and Management, 3(57):21-27.
Matsudo, M. C., Bezerra, R. P., Sato, S., Perego, P., Converti, A., & Carvalho, J. C. M. (2009). Repeated fed-batch cultivation of Arthrospira (Spirulina) platensis using urea as nitrogen source. Biochemical Engineering Journal, 43(1):52-57.
Pinton, R., Tomasi, N., & Zanin, L. (2016). Molecular and physiological interactions of urea and nitrate uptake in plants. Plant Signaling & Behavior, 11(1):e1076603.
Podevin, M., De Francisci, D., Holdt, S. L., & Angelidaki, I. (2015). Effect of nitrogen source and acclimatization on specific growth rates of microalgae determined by a high-throughput in vivo microplate autofluorescence method. Journal of Applied Phycology, 27(4):1415-1423.
Qiu, R., Gao, S., Lopez, P. A., & Ogden, K. L. (2017). Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Research, 28:192-199.
Ramanna, L., Guldhe, A., Rawat, I., & Bux, F. (2014). The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresource Technology, 168:127-135.
Raven, J. A., & Giordano, M. (2016). Combined nitrogen. In M. Borowitzka, J. Beardall, & J. Raven (Ed.), The physiology of microalgae. (pp. 143-154). Switzerland: Springer International Publishing.
Ribeiro, D. M., Roncaratti, L. F., Possa, G. C., Garcia, L. C., Cançado, L. J., Williams, T. C. R., & Brasil, B. dos S. A. F. (2020). A low-cost approach for Chlorella sorokiniana production through combined use of urea, ammonia and nitrate based fertilizers. Bioresource Technology Reports, 9:100354.
Rodrigues, M. S., Ferreira, L. S., Converti, A., Sato, S., & de Carvalho, J. C. M. (2011). Influence of ammonium sulphate feeding time on fed-batch Arthrospira (Spirulina) platensis cultivation and biomass composition with and without pH control. Bioresource Technology, 102(11):6587-6592.
Safafar, H., Ní¸rregaard, P. U., Ljubic, A., Mí¸ller, P., Holdt, S. L., & Jacobsen, C. (2016). Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. Journal of Marine Science and Engineering, 4(48):1-15.
Scherholz, M. L., & Curtis, W. R. (2015). Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnology, 13(39):1-6.
Singh, S. P., & Singh, P. (2014). Effect of CO2 concentration on algal growth: A review. Renewable and Sustainable Energy Reviews, 38:172-179.
Takaichi, S. (2011). Carotenoids in algae: Distributions, biosyntheses and functions. Marine Drugs, 9(6):1101-1118.
Wang, J., Sommerfeld, M. R., Lu, C., & Hu, Q. (2013). Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae, 28(2):193-202.
Xin, L., Hong-ying, H., Ke, G., & Ying-xue, S. (2010). Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresource Technology, 101(14):5494-5500.
Xu, N., Zhang, X., Fan, X., Han, L., & Zeng, C. (2001). Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). Journal of Applied Phycology, 13:463-469.
Xu, Y., Ibrahim, I. M., & Harvey, P. J. (2016). The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiology and Biochemistry, 106:305-315.
Yaakob, Z., Ali, E., Zainal, A., Mohamad, M., & Takriff, M. S. (2014). An overview: biomolecules from microalgae for animal feed and aquaculture. Journal of Biological Research – Thessaloniki, 21(6):1-10.
Young, E. B., & Beardall, J. (2003). Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. Journal of Phycology, 39(5):897-905.
Yuan, C., Xu, K., Sun, J., Hu, G. R., & Li, F. L. (2018). Ammonium, nitrate, and urea play different roles for lipid accumulation in the nervonic acid”producing microalgae Mychonastes afer HSO-3-1. Journal of Applied Phycology, 30(2):793-801.