
Date Log
Copyright (c) 2025 Jurnal Ilmiah Perikanan dan Kelautan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
1. Copyright of the article is transferred to the journal, by the knowledge of the author, whilst the moral right of the publication belongs to the author.
2. The legal formal aspect of journal publication accessibility refers to Creative Commons Atribusi-Non Commercial-Share alike (CC BY-NC-SA), (https://creativecommons.org/licenses/by-nc-sa/4.0/)
3. The articles published in the journal are open access and can be used for non-commercial purposes. Other than the aims mentioned above, the editorial board is not responsible for copyright violation
The manuscript authentic and copyright statement submission can be downloaded ON THIS FORM.
Effect of Crossbreeding on Fecundity, Growth Performance, and Heterosis of Black Tilapia, Red Tilapia, and Mozambique Tilapia Reared in Earthen Ponds in West Java, Indonesia
Corresponding Author(s) : Adam Robisalmi
Jurnal Ilmiah Perikanan dan Kelautan, Vol. 17 No. 1 (2025): JURNAL ILMIAH PERIKANAN DAN KELAUTAN
Abstract
Graphical Abstract
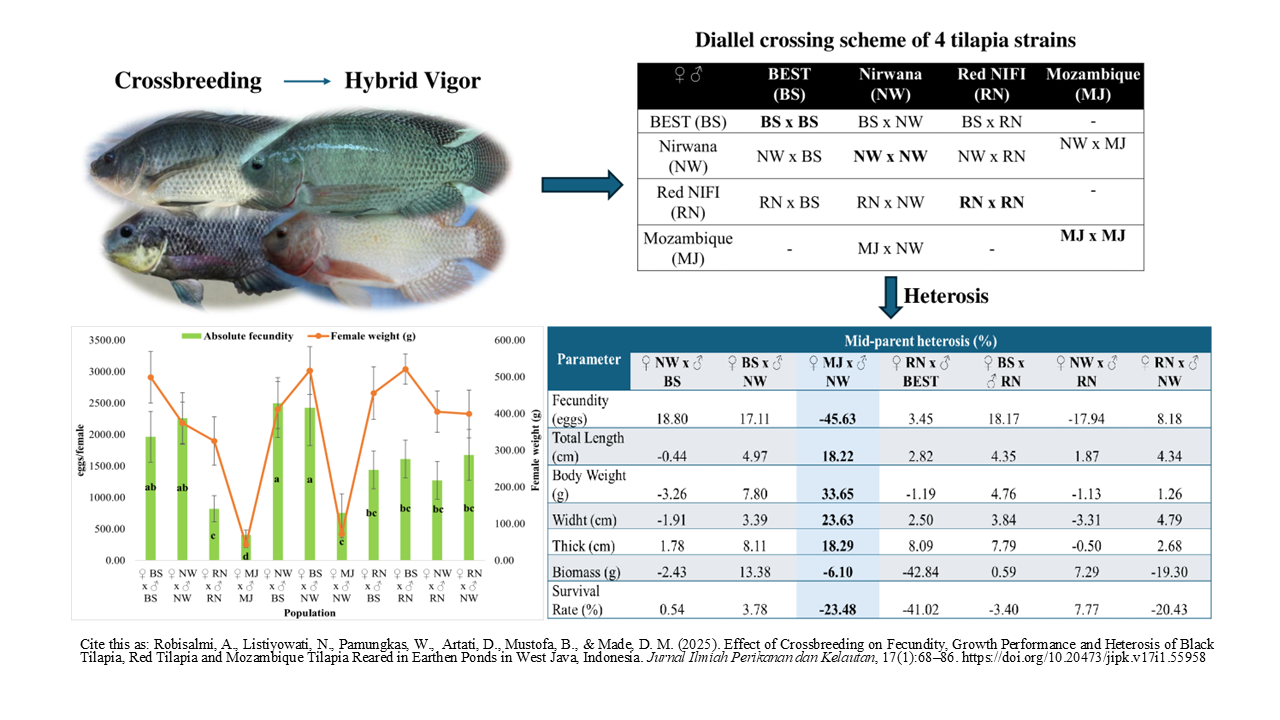
Highlight Research
- The crossbred performance of the four tilapia strains showed diverse heterosis values, with some being better, worse, or the same as their parents.
- Crossbred of black tilapia produced the best growth and fecundity performance.
- A hybrid of Mozambique tilapia and black tilapia showed the highest mid-parent heterosis value on growth traits but produced negative heterosis on fecundity, biomass, and survival traits.
- Crossbred of black tilapia and Mozambique tilapia have the potential to be used as candidates for cultivation and performance improvement through selection, although there are depressions and crossover advantages that are not prominent.
Abstract
Increased tilapia production is challenged by genetic decline. Hybridization efforts for performance improvement through a selection of the best parent and strain pairs were a promising option. The objective of this study was to evaluate the crossing of black tilapia, red tilapia, and Mozambique tilapia against the performance of fecundity, growth, and survival and estimate the value of heterosis. The experimental design used a completely randomized design with 3 replications with the treatment of different populations of crosses. The rearing activities were carried out in earthen ponds for 150 days with a stocking density of 10 fish/m2. The parameters observed included egg fecundity, growth, survival, and the value of heterosis. The results showed that the fecundity and growth values of crossbred black tilapia were significantly higher than others (p<0.05). The highest survival rate was shown by crossing pure strains of red tilapia. The hybrid of Mozambique tilapia and black tilapia (♀ MJ x ♂ NW) showed the highest mid-parent heterosis value on growth characters but produced negative heterosis on characters, fecundity, biomass, and survival. Overall, the crossbred of black tilapia (♀BS x ♂ NW) performed better than the inbred strains, with positive mid-parent heterosis in all characters measured. These results indicate that crossbreeding has the potential to be used as a candidate for cultivation and performance improvement through selection, although there were depressions, and the superiority of the crosses was not prominent.
Keywords
Download Citation
Endnote/Zotero/Mendeley (RIS)BibTeX
- Abdel-Tawwab, M., Ahmad, M. H., Khattab, Y. A. E., & Shalaby, A. M. E. (2010). Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture, 298(3–4):267-274.
- Abwao, J., Kyule, D., Junga, J. O., Barasa, J. E., & Sigana, D. A. (2023). On‐farm growth performance of different strains of tilapia, Oreochromis niloticus reared in earthen ponds. Aquaculture, Fish and Fisheries, 3(3):247-255.
- Akian, D. D., Yao, K., Clota, F., Lozano, P., Baroiller, J. F., Chatain, B., & Bégout, M. L. (2017). Reproductive behaviour of two tilapia species (Oreochromis niloticus, Linné, 1758; Sarotherodon melanotheron, Rüppel, 1852) in freshwater intra and interspecific pairing context. Applied Animal Behaviour Science, 193(8):104-113.
- Almeida, D. B., da Costa, M. A. P., Bassini, L. N., Calabuig, C. I. P., Moreira, C. G. A., Rodrigues, M. D. N., Pérez, H. J., Tavares, R. A., Varela, A. S., & Moreira, H. L. M. (2013). Reproductive performance in female strains of Nile tilapia, Oreochromis niloticus. Aquaculture International, 21(6):1291-1300.
- Altinok, I., Ozturk, R. C., Capkin, E., & Kalayci, G. (2020). Experimental crossbreeding reveals variation in growth among brown trout (Salmo trutta) strains and their reciprocal crossbreeds. Aquaculture, 521(8):1-9.
- Arifin, O. Z., Imron, Aseppendi, Hendri, A., Muslim, N., & Yani, A. (2017). Intraspecific hybridization between two populations of Galunggung giant gourami (Osphronemus goramy Lacepede, 1801). Jurnal Riset Akuakultur, 12(4):315-323.
- Bartie, K. L., Taslima, K., Bekaert, M., Wehner, S., Syaifudin, M., Taggart, J. B., de Verdal, H., Rosario, W., Muyalde, N., Benzie, J. A. H., McAndrew, B. J., & Penman, D. J. (2020). Species composition in the Molobicus hybrid tilapia strain. Aquaculture, 526(13):1-7.
- Basavaraja, N., & Raghavendra, C. H. (2017). Hormonal sex reversal in red tilapia (Oreochromis niloticus and Oreochromis mossambicus) and inheritance of body colour in O. mossambicus and red tilapia: Implications for commercial farming. Aquaculture International, 25(3):1317-1331.
- Bentsen, H. B., Eknath, A. E., Palada-de Vera, M. S., Danting, J. C., Bolivar, H. L., Reyes, R. A., Dionisio, E. E., Longalong, F. M., Circa, A. V., Tayamen, M. M., & Gjerde, B. (1998). Genetic improvement of farmed tilapias: Growth performance in a complete diallel cross experiment with eight strains of Oreochromis niloticus. Aquaculture, 160(1–2):145-173.
- Boyd, C. E. (2015). Water quality: An introduction. 2nd Edition. Springer.
- Bradbeer, S. J., Harrington, J., Watson, H., Warraich, A., Shechonge, A., Smith, A., Tamatamah, R., Ngatunga, B. P., Turner, G. F., & Genner, M. J. (2019). Limited hybridization between introduced and critically endangered indigenous tilapia fishes in northern Tanzania. Hydrobiologia, 832(1):257-268.
- Cao, J., Yang, N., Liu, Z., Lu, M., Gao, F., Ke, X., Wang, M., & Yi, M. (2021). Distant hybridization and gynogenesis between Nile tilapia Oreochromis niloticus and Jaguar cichlid Parachromis managuensis. Animal Reproduction Science, 232(9):1-12.
- Chen, J., Fan, Z., Tan, D., Jiang, D., & Wang, D. (2018). A review of genetic advances related to sex control and manipulation in tilapia. Journal of the World Aquaculture Society, 49(2):277-291.
- Chen, S., Tian, Y., Li, Z., Liu, Y., Li, Z., Duan, P., Li, L., Wang, X., Wang, L., He, X., Zhao, X., Li, W., & Wang, Q. (2023). Heterosis in growth and low temperature tolerance in Jinhu grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂). Aquaculture, 562(1):1-13.
- Correia, D., Escarcega-Miranda, B., Barreto-Curiel, F., Mata-Sotres, J., del Rio-Zaragoza, O., Viana, M. T., & Rombenso, A. N. (2019). Growth performance and body composition of hybrid red tilapia (Oreochromis mossambicus × O. aureus) fed with different protein levels raised in saltwater. Latin American Journal of Aquatic Research, 47(5):853-859.
- Coward, K., & Bromage, N. R. (2000). Reproductive physiology of female tilapia broodstock. Reviews in Fish Biology and Fisheries, 10(1):1-25.
- Crespel, A., Audet, C., Bernatchez, L., & Garant, D. (2012). Effects of rearing environment and strain combination on heterosis in brook trout. North American Journal of Aquaculture, 74(2):188-198.
- Cruz, P., & Ibarra, A. M. (1997). Larval growth and survival of two catarina scallop (Argopecten circularis, Sowerby, 1835) populations and their reciprocal crosses. Journal of Experimental Marine Biology and Ecology, 212(1):95-110.
- Dai, P., Wang, H., Xiao, G., & Liu, B. (2014). Combining ability and heterosis analysis over two environments in a diallel cross of three families of the clam Meretrix meretrix. Acta Oceanologica Sinica, 33(10):37-42.
- De Donato, M., Manrique, R., Ramirez, R., Mayer, L., & Howell, C. (2005). Mass selection and inbreeding effects on a cultivated strain of Penaeus (Litopenaeus) vannamei in Venezuela. Aquaculture, 247(1–4):159-167.
- De Verdal, H., Rosario, W., Vandeputte, M., Muyalde, N., Morissens, P., Baroiller, J. F., & Chevassus, B. (2014). Response to selection for growth in an interspecific hybrid between Oreochromis mossambicus and O. niloticus in two distinct environments. Aquaculture, 430(8):159-165.
- Delomas, T. A., Gomelsky, B., Vu, N., Campbell, M. R., & Novelo, N. D. (2019). Single-nucleotide polymorphism discovery and genetic variation in YY-male and mixed-sex strains of Nile tilapia available in the United States. North American Journal of Aquaculture, 81(3):183-188.
- Desprez, D., Briand, C., Hoareau, M. C., Mélard, C., Bosc, P., & Baroiller, J. F. (2006). Study of sex ratio in progeny of a complex Oreochromis hybrid, the Florida red tilapia. Aquaculture, 251(2–4):231-237.
- El-Araby, D. A., Amer, S. A., & Khalil, A. A. (2020). Effect of different feeding regimes on the growth performance, antioxidant activity, and health of Nile tilapia, Oreochromis niloticus. Aquaculture, 528(15):1-10.
- El-Sayed, A.-F. M. (2020a). The role of tilapia culture in rural development. Tilapia Culture, 275-295.
- El-Sayed, A.-F. M. (2020b). Environmental requirements. Tilapia Culture, 47-67.
- Falconer, D. S., & Mackay, T. F. C. (1996). Introduction to quantitative genetics. 4th Ed. England: Longman.
- Fehr, W. R. (1991). Principles of cultivar development, theory and technique. New York: Macmillan Publishing Company.
- Goddard, S. (1996). Feeds in intensive aquaculture. In S. Goddard. Feed management in intensive aquaculture. (pp. 1-22). Springer.
- Gomelsky, B. (2011). Fish genetics: Theory and practice. Saarbrücken, Germany: VDM Verlag Dr. Mueller GmbH & Co.
- Granier, S., Audet, C., & Bernatchez, L. (2011). Heterosis and outbreeding depression between strains of young-of-the-year brook trout (Salvelinus fontinalis). Canadian Journal of Zoology, 89(3):190-198.
- Githukia, C. M., Ogello, E. O., Kembenya, E. M., Achieng, A. O., Obiero, K. O., & Munguti, J. M. (2015). Comparative growth performance of male monosex and mixed sex nile tilapia (Oreochromis niloticus l.) reared in earthen ponds. Ribarstvo, Croatian Journal of Fisheries, 73(1):20-25.
- Hallauer, A. R., Carena, M. J., & Filho, J. B. M. (2010). Heterosis. In: Quantitative Genetics in Maize Breeding. Part of the book series: Handbook of Plant Breeding. Vol 6. (pp. 477-529). New York: Springer.
- Hamzah, A., Nguyen, N. H., Mekkawy, W., Khaw, H. L., Yee, H. Y., Abu Bakar, K. R., Ponzoni, R. W., & Mohd Nor, S. A. (2016). Genetic parameters and correlated responses in female reproductive traits in the GIFT strain. Aquaculture Research, 47(5):1488-1498.
- Hasan, M & Soto, D. (2017). Improving feed conversion ratio and its impact on reducing greenhouse gas. Rome: Food and Agriculture Organization of The United Nations.
- Herawati,V. E., Susilo, A., Pinandoyo, Hutabarat, J., Sugianto, D. N., Wirasatriya, A., & Karnaradjasa, O. (2019). Optimization of fish meal substitution with maggot meal (Hermetia Illucens) for growth and feed utilization efficiency of juvenile Litopenaeus vannamei. Asian Journal of Microbiology, Biotechnology & Environmental Sciences, 21(2):284-297.
- Hernández-Gurrola, J. A., Naranjo-Páramo, J., Vargas-Mendieta, M., Cruz-Hernández, P., Villarreal-García, A., Mora-Castrejón, G., & Villarreal-Colmenares, H. (2020). Effect of crossbreeding three divergent populations on the juvenile production and rearing performance of the redclaw crayfish Cherax quadricarinatus. Aquaculture, 527(14):1-10.
- Hovick, S. M., & Whitney, K. D. (2014). Hybridisation is associated with increased fecundity and size in invasive taxa: Meta-analytic support for the hybridisation-invasion hypothesis. Ecology Letters, 17(11):1464-1477.
- Huxel, G. R. (1999). Rapid displacement of native species by invasive species: Effects of hybridization. Biological Conservation, 89(2):143-152.
- Jiang, G., Li, Q., Xu, C., Liu, S., Kong, L., & Yu, H. (2021). Reciprocal hybrids derived from Crassostrea gigas and C. angulata exhibit high heterosis in growth, survival and thermotolerance in northern China. Aquaculture, 545(16):1-9.
- Jiang, G., Li, Q., & Xu, C. (2022). Growth, survival and gonad development of two new types of reciprocal triploid hybrids between Crassostrea gigas and C. angulata. Aquaculture, 559(15):1-11.
- Joshi, R., Woolliams, J. A., Meuwissen, T. H. E., & Gjøen, H. M. (2018). Maternal, dominance and additive genetic effects in Nile tilapia; influence on growth, fillet yield and body size traits. Heredity, 120(5):452-462.
- Khaw, H. L., Ponzoni, R. W., Yee, H. Y., bin Aziz, M. A., & Bijma, P. (2016). Genetic and non-genetic indirect effects for harvest weight in the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture, 450(1):154-161.
- Kong, L., Song, S., & Li, Q. (2017). The effect of interstrain hybridization on the production performance in the Pacific oyster Crassostrea gigas. Aquaculture, 472(7):44-49.
- Koolboon, U., Koonawootrittriron, S., Kamolrat, W., & Na-Nakorn, U. (2014). Effects of parental strains and heterosis of the hybrid between Clarias macrocephalus and Clarias gariepinus. Aquaculture, 424–425(5):131-139.
- Krasnovyd, V., Vetešník, L., & Šimková, A. (2020). Distribution of host-specific parasites in hybrids of phylogenetically related fish: The effects of genotype frequency and maternal ancestry? Parasites and Vectors, 13(1):1-11.
- In, V. Van, Sang, V. Van, O’Connor, W., Van, P. T., Dove, M., Knibb, W., & Nguyen, N. H. (2017). Are strain genetic effect and heterosis expression altered with culture system and rearing environment in the Portuguese oyster (Crassostrea angulata)? Aquaculture Research, 48(8):4058-4069.
- Lago, A. de A., Rezende, T. T., Dias, M. A. D., Freitas, R. T. F. de, & Hilsdorf, A. W. S. (2017). The development of genetically improved red tilapia lines through the backcross breeding of two Oreochromis niloticus strains. Aquaculture, 472(8):17-22.
- Lal, M. M., Waqairatu, S. S., Zenger, K. R., Nayfa, M. G., Pickering, T. D., Singh, A., & Southgate, P. C. (2021). The GIFT that keeps on giving? A genetic audit of the Fijian genetically improved farmed tilapia (GIFT) broodstock nucleus 20 years after introduction. Aquaculture, 537(8):1-18.
- Li, Z., Coffey, L., Garfin, J., Miller, N. D., White, M. R., Spalding, E. P., De Leon, N., Kaeppler, S. M., Schnable, P. S., Springer, N. M., & Hirsch, C. N. (2018). Genotype-by-environment interactions affecting heterosis in maize. PLoS One, 13(1):1-16.
- Liang, Y., Xu, C., & Li, Q. (2023). Growth, survival and color segregation of F2 hybrids between selected “Haida no.1” and orange-shell lines of the Pacific oyster. Aquaculture, 574(14):1-8.
- Liang, Y., Xu, C., & Li, Q. (2023). Heterosis and genetic diversity of intraspecific hybrids crosses between two selected lines of the Pacific oyster Crassostrea gigas. Aquaculture, 569(9):1-9.
- Lind, C. E., Ponzoni, R. W., Nguyen, N. H., & Khaw, H. L. (2012). Selective breeding in fish and conservation of genetic resources for aquaculture. Reproduction in Domestic Animals, 47(4):255–263.
- Liu, S. J. (2010). Distant hybridization leads to different ploidy fishes. Science China Life Sciences, 53(4):416-425.
- Lozano, C. A., Gjerde, B., Ødegård, J., Rye, M., & Dinh, T. (2014). Heritability estimates for male proportion in hybrids between Nile tilapia females (Oreochromis niloticus ) and blue tilapia males (Oreochromis aureus). Aquaculture, 430(8):66-73.
- Lu, B., Liang, G., Xu, M., Wang, C., Tan, D., Tao, W., Sun, L., & Wang, D. (2022). Production of all male amelanotic red tilapia by combining MAS-GMT and tyrb mutation. Aquaculture, 546(1):1-11.
- Maluwa, A. O., & Gjerde, B. (2006a). Genetic evaluation of four strains of Oreochromis shiranus for harvest body weight in a diallel cross. Aquaculture, 259(1–4):28-37.
- Maluwa, A. O., & Gjerde, B. (2006b). Estimates of the strain additive, maternal and heterosis genetic effects for harvest body weight of an F2 generation of Oreochromis shiranus. Aquaculture, 259(1–4):38-46.
- Marengoni, N. G., Onoue, Y., & Oyama, T. (1998). Offspring growth in a diallel crossbreeding with three strains of Nile tilapia Oreochromis niloticus. Journal of the World Aquaculture Society, 29(1):114-119.
- MMAF. (2022). Marine and fisheries in figures 2022. Jakarta: The Center for Data, Statistics and Information. Ministry of Marine Affairs and Fisheries of the Republic of Indonesia.
- Mashaii, N., Rajabipour, F., Mohammadi, M., Sarsangi, H., Bitaraf, A., Hossein-Zadeh, H., & Sharif-Rohani, M. (2016). Reproduction of Nile tilapia, Oreochromis niloticus in brackish water. Journal of Applied Aquaculture, 28(1):1-8.
- Mbiru, M., & Mchele, S. (2015). Comparative performance of mixed-sex and hormonal- sex-reversed Nile tilapia Oreochromis niloticus. Aquaculture International, 24(2):557-566.
- Mengistu, S. B., Mulder, H. A., Benzie, J. A. H., Khaw, H. L., Megens, H. J., Trinh, T. Q., & Komen, H. (2020). Genotype by environment interaction between aerated and non-aerated ponds and the impact of aeration on genetic parameters in Nile tilapia (Oreochromis niloticus). Aquaculture, 529(16):1-10.
- Moses, M., Chauka, L. J., de Koning, D. J., Palaiokostas, C., & Mtolera, M. S. P. (2021). Growth performance of five different strains of Nile tilapia (Oreochromis niloticus) introduced to Tanzania reared in fresh and brackish waters. Scientific Reports, 11(1):1-12.
- Mtaki, K., Limbu, S. M., Mmochi, A. J., & Mtolera, M. S. P. (2022). Hybrids production as a potential method to control prolific breeding in tilapia and adaptation to aquaculture climate-induced drought. Aquaculture and Fisheries, 7(6):647-652.
- NRC [National Research Council]. (1983). Nutrient requirements of warmwater fishes. Sub Committee on Warmwater Fish Nutrition. Committee on Animal Nutrition Board on Agriculture and Renewable Resources. Washington DC: National Academy Science.
- Neira, R., García, X., Paul, J., Filp, M., Manuel, J., & María, A. (2016). Evaluation of the growth and carcass quality of diallel crosses of four strains of Nile tilapia ( Oerochromis niloticus ). Aquaculture, 451(12):213-222.
- Novelo, N. D., Gomelsky, B., Coyle, S. D., & Kramer, A. G. (2021). Evaluation of growth, sex (male proportion; sexual dimorphism), and color segregation in four cross combinations of different strains of XX female and YY male Nile Tilapia. Journal of the World Aquaculture Society, 52(2):445-456.
- Nugroho, E., Rustadi, Priyanto, D., Sulistyo, H., Susila, Sunaryo & Wasito, B. (2014). Lost of genetic variation of genetically improved strain of “Cangkringan” red tilapia monitored in F-4 detected by DNA Marker. Jurnal Riset Akuakultur, 9(1):25-30.
- Nzohabonayo, E., Manyala, J., Kang’ombe, J., & Kassam, D. (2021). Effect of hybridization on reproductive performance of Oreochromis karongae, Oreochromis shiranus and Oreochromis mossambicus. Aquaculture Research, 52(1):302-308.
- Omasaki, S. K., Charo-karisa, H., Kahi, A. K., & Komen, H. (2016). Genotype by environment interaction for harvest weight, growth rate and shape between monosex and mixed sex Nile tilapia (Oreochromis niloticus). Aquaculture, 458(9):75-81.
- Osure, G. O., & Phelps, R. P. (2006). Evaluation of reproductive performance and early growth of four strains of Nile tilapia (Oreochromis niloticus L) with different histories of domestication. Aquaculture, 253(1–4):485-494.
- Piwpong, N., Chiayvareesajja, J., & Chiayvareesajja, S. (2017). Growth and survival of a diallel cross for five strains of climbing perch (Anabas testudineus Bloch, 1792) in Thailand. Agriculture and Natural Resources, 50(5):351-356.
- Pongthana, N., Hong, N., & Ponzoni, R. W. (2010). Comparative performance of four red tilapia strains and their crosses in fresh and saline water environments. Aquaculture, 308(12):S109-S114.
- Ponzoni, R. W., Nguyen, N. H., Khaw, H. L., Hamzah, A., Bakar, K. R. A., & Yee, H. Y. (2011). Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the World Fish Center with the GIFT strain. Reviews in Aquaculture, 3(1):27-41.
- Poolsawat, L., Yu, Y., Li, X., Zhen, X., Yao, W., Wang, P., Luo, C., & Leng, X. (2022). Efficacy of phytogenic extracts on growth performance and health of tilapia (Oreochromis niloticus × O. aureus). Aquaculture and Fisheries, 7(4):411-419.
- Qin, Y., Li, X., Liao, Q., Li, J., Ma, H., Mo, R., Zhang, Y., & Yu, Z. (2021). Comparative study on the growth, survival, gonad development and trait segregation of F2 hybrids and their grandparent species (Crassostrea ariakensis and C. hongkongensis). Aquaculture, 541(12):1-9.
- Radona, D., Subagja, J., Otong, D., Arifin, Z., Penelitian, B., Budidaya, P., & Tawar, A. (2016). Performance of reproductive aspects of broodstock and growth of seed of Tor soro, Tor douronensis, and their reciprocal crosses. Jurnal Riset Akuakultur, 10(3):335-343.
- Rahman, M. A., Arshad, A., Marimuthu, K., Ara, R., & Amin, S. M. N. (2013). Inter-specific hybridization and its potential for aquaculture of fin fishes. Asian Journal of Animal and Veterinary Advances. 8(2):139-153.
- Rapatsa, M. M., & Moyo, N. A. G. (2017). Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquaculture Reports, 5(1):18-26.
- Rasidi., Nugroho, E., Radona, D., Emmawatie, L., & Ardi, I. (2015). Heterosis value and growth performance of four populations of tilapia (Oreochromis niloticus) resulting from reciprocal breeding. Prosiding Forum Teknologi Akuakultur 2015: 183–190.
- Ricker, W. E. (1979). Growth rates and models. Fish Physiology, 8(1):677-743.
- Ridha, M. T. (2012). Preliminary observations on growth and survival of Oreochromis spilurus x GIFT Oreochromis niloticus F 1 reciprocal hybrids in fresh and seawater. Aquaculture Research, 45(3):1-9.
- Robisalmi, A., Gunadi, B., & Setyawan, P. (2020). Evaluation of Growth Performance and Heterosis of hybridization between female nile tilapia (Oreochromis niloticus) with male blue tilapia (Oreochromis aureus) F2 on Hipersalinity Brakish water Pond. Berita Biologi, 19(1):1-11.
- Robisalmi, A., Alipin, K., & Gunadi, B. (2021). Growth performance and microstructure in the muscle fiber of red tilapia ( Oreochromis spp.) under different cycles of fasting and refeeding. AACL Bioflux, 14(3):1498-1512.
- Rossato, K. A., Mauerwerk, M. T., Balen, R. E., Zadinelo, I. V., & da Silva, L. C. R. (2022). Interaction between weight and strain of Nile tilapia broodstocks in relation to growth and reproductive performance. Aquaculture, 559(15):1-5.
- Russell, D. J., Thuesen, P. A., & Thomson, F. E. (2012). Reproductive strategies of two invasive tilapia species Oreochromis mossambicus and Tilapia mariae in northern Australia. Journal of Fish Biology, 80(6):2176-2197.
- Saleky, D., Sianturi, R., Dailami, M., & Kusuma, A. B. (2021). Molecular study of Oreochromis spp. from Merauke Waterland-Papua, based on mitochondrial DNA cytochrome oxidase subunit I fragment gene. Jurnal Perikanan Universitas Gadjah Mada, 23(1):37-43.
- Sarker, B. S., Sabuz, M. S. R., Azom, M. G., Easmin, N., Ali, A., Alam, M. S., & Islam, M. S. (2023). Genotype responsive growth performance and salinity tolerance of tilapia hybrid (Oreochromis niloticus × O. mossambicus). Aquaculture, 575(15):1-9.
- Sarmento, N. L. A. F., Martins, E. F. F., Costa, D. C., Mattioli, C. C., da Costa Julio, G. S., Figueiredo, L. G., Luz, M. R., & Luz, R. K. (2018). Reproductive efficiency and egg and larvae quality of Nile tilapia fed different levels of vitamin C. Aquaculture, 482(1):96-102.
- Shivaramu, S., Vuong, D. T., Havelka, M., Šachlová, H., Lebeda, I., Kašpar., V., & Flajšhans, M. (2019). Influence of interspecific hybridization on fitness-related traits in Siberian sturgeon and Russian sturgeon. Czech Journal of Animal Science, 64(2):78-88.
- Silva, A. C. F., Filho, R. A. C. C., Ventura, A. S., Nunes, A. L., Laice, L. M., Ribeiro, R. P., Oliveira, C. A. L., Almeida, L. C., Barbosa, P. T. L., & Povh, J. A. (2020). Reproductive traits in different Nile tilapia genetic groups [Caracteristicas reprodutivas em diferentes grupos geneticos de tilápia do Nilo]. Arquivo Brasileiro de Medicina Veterinári e Zootecnia, 72(5):1797-1804.
- Silva, A. C. C., Corrêa Filho, R. A. C., Fornari, D. C., Abreu, J. S. de, Bignardi, A. B., Severino, M. de F. G., Amorim, L. F. dos S., Albuquerque, L. V., Carneiro, I. L., & Povh, J. A. (2022). Production of tambaqui and of the tambatinga and tambacu hybrids: Performance, morphometric traits, and body yield. Aquaculture, 554(10):1-7.
- Šimková, A., Janáč, M., Hyršl, P., Krasnovyd, V., & Vetešník, L. (2021). Vigour-related traits and immunity in hybrids of evolutionary divergent cyprinoid species: advantages of hybrid heterosis? Journal of Fish Biology, 98(4):1155-1171.
- Snake, M., Maluwa, A., Zidana, H., Chigwechokha, P., & Simwaka, M. (2020). Production of a predominantly male tilapia progeny using two Malawian tilapias, Oreochromis shiranus and Oreochromis karongae. Aquaculture Reports, 16(1):1-5.
- Söderquist, L., Broberg, A., Rosenberg, V., & Sletvold, N. (2020). Predicting heterosis and inbreeding depression from population size and density to inform management efforts. Journal of Applied Ecology, 57(8):1459-1468.
- Subekti, S., Arief, M., & Yudha, G. C. P. (2016). Silage substitution chemically solid waste surimi fish swanggi (Priacanthus macracanthus) on to retention protein fish meal and retention fat tilapia (Oreochromis niloticus). Jurnal Ilmiah Perikanan dan Kelautan, 8(2):77-83.
- Sun, C., Dong, J., Li, W., Tian, Y., Hu, J., & Ye, X. (2022). Response to four generations of selection for growth performance traits in mandarin fish (Siniperca chuatsi). Aquaculture, 548(3):1-6.
- Tave, D. (1995). Selective breeding programmes for medium-sized fish farms. In FAO. Fisheries Technical Paper. Vol. 352.
- Tave, D., Jayaprakas, V., & Smitherman O. R. (1990). Effects of Intraspecific Hybridization in. Journal of the World Aquaculture Society, 21(3):201-204.
- Thoa, N. P., Ninh, N. H., Hoa, N. T., Knibb, W., Diep, N. H., & Nguyen, N.H. (2016). Additive genetic and heterotic effects in a 4 x 4 complete diallel cross-population of Nile tilapia (Oreochromis niloticus, Linnaeus, 1758) reared in different water temperature environments in Northern Vietnam. Aquacultre Research, 47(3):708-720.
- Thoa, N. P., Hamzah, A., & Nguyen, N. H. (2017). Genetic variation and correlated changes in reproductive performance of a red tilapia line selected for improved growth over three generations. Animal Reproduction Science, 184(9):94-101.
- Thodesen Da-Yong Ma, J., Rye, M., Wang, Y. X., Yang, K. S., Bentsen, H. B., & Gjedrem, T. (2011). Genetic improvement of tilapias in China: Genetic parameters and selection responses in growth of Nile tilapia (Oreochromis niloticus) after six generations of multi-trait selection for growth and fillet yield. Aquaculture, 322–323(18):51-64.
- Thongprajukaew, K., Kovitvadhi, S., Kovitvadhi, U., & Preprame, P. (2017). Effects of feeding frequency on growth performance and digestive enzyme activity of sex-reversed Nile tilapia, Oreochromis niloticus (Linnaeus, 1758). Agriculture and Natural Resources, 51(4):292-298.
- Todesco, M., Pascual, M. A., Owens, G. L., Ostevik, K. L., Moyers, B. T., Hübner, S., Heredia, S. M., Hahn, M. A., Caseys, C., Bock, D. G., & Rieseberg, L. H. (2016). Hybridization and extinction. Evolutionary Applications, 9(7):892-908.
- Trinh, T. Q., Agyakwah, S. K., Khaw, H. L., Benzie, J. A. H., & Attipoe, F. K. Y. (2021). Performance evaluation of Nile tilapia (Oreochromis niloticus) improved strains in Ghana. Aquaculture, 530(1):1-6.
- Trong, T. Q., Van Arendonk, J. A. M., & Komen, H. (2013). Genetic parameters for reproductive traits in female Nile tilapia (Oreochromis niloticus): II. Fecundity and fertility. Aquaculture, 416–417(16):72-77.
- Tuan Harith, Z., Mohd Sukri, S., Remlee, N. F. S., Mohd Sabir, F. N., & Zakaria, N. N. A. (2022). Effects of dietary astaxanthin enrichment on enhancing the colour and growth of red tilapia, Oreochromis sp. Aquaculture and Fisheries, 9(1):52-56.
- Valentin, F. N., do Nascimento, N. F., da Silva, R. C., Tsuji, E. A., Paes, M. C. F., Koberstein, T. C. R. D., & Nakaghi, L. S. O. (2015). Maternal age influences on reproductive rates in Nile tilapia (Oreochromis niloticus). Revista Brasileira de Zootecnia, 44(4):161-163.
- Wang, C. H., Li, S. F., Liu, Z. G., Xiang, S. P., Wang, J., Pang, Z. Y., & Duan, J. P. (2006). Developmental quantitative genetic analysis of body weight and morphological traits in red common carp, Cyprinus carpio L. Aquaculture, 251(2–4):219–230.
- Wang, C., & Li, Z. (2010). Improvement in production traits by mass spawning type crossbreeding in bay scallops. Aquaculture, 299(1–4):51-56.
- Wang, S., Tang, C., Tao, M., Qin, Q., Zhang, C., & Luo, K. (2019). Establishment and application of distant hybridization technology in fish. Science China Life Sciences, 62(1):22-45.
- Whitlock, M. C., Ingvarsson, P. K., & Hatfield, T. (2000). Local drift load and the heterosis of interconnected populations. Heredity, 84(4):452-457.
- Workakegn, B. K. (2019). Establishment of base population for long-term genetic improvement of Nile tilapia in Ethiopia. PhD dissertation. Norway: NMBU.
- Xiao, Q., Shen, Y., Gan, Y., Wang, Y., Zhang, J., Huang, Z., You, W., Luo, X., & Ke, C. (2022). Three-way cross hybrid abalone exhibit heterosis in growth performance, thermal tolerance, and hypoxia tolerance. Aquaculture, 555(11):1-9.
- Xing, Q., Yang, Z., Zhu, X., Liu, J., Huang, X., Hu, J., & Bao, Z. (2022). Interspecific hybridization between Patinopecten yessoensis (♀) and (♂) with heterosis in growth and temperature tolerance. Aquaculture, 547(2):1-14.
- Xu, K., Duan, W., Xiao, J., Tao, M., Zhang, C., Liu, Y., & Liu, S. J. (2015). Development and application of biological technologies in fish genetic breeding. Science China Life Sciences, 58(2):187-201.
- Yamasaki, L. S., Iwai, T., Klinger-Bowen, R. E. C., Weese, D. A., Fowler, C. E., Yacoub, J. L., & Wong, M. A. (2022). Identification of Nile tilapia (Oreochromis niloticus) and its hybrids in natural environments in Hawaii. Aquaculture, 550(6):1-5.
- Yan, L., Su, J., Wang, Z., Zhang, Y., Yan, X., & Yu, R. (2018). Growth performance and biochemical composition of the oysters Crassostrea sikamea, Crassostrea angulata and their hybrids in Southern China. Aquaculture Research, 49(2):1020-1028.
- Yoshida, G. M., de Oliveira, C. A. L., Kunita, N. M., Rizzato, G. S., & Ribeiro, R. P. (2015). Reproduction performance of female Nile tilapia under different environments and age classes. Acta Scientiarum - Animal Sciences, 37(3):221-226.
- Yu, D., Gu, X., Zhang, S., Dong, S., Miao, H., Gebretsadik, K., & Bo, K. (2021). Molecular basis of heterosis and related breeding strategies reveal its importance in vegetable breeding. Horticulture Research, 8(1):1-17.
- Zak, T., Deshev, R., Benet-Perlberg, A., Naor, A., Magen, I., Shapira, Y., Ponzoni, R. W., & Hulata, G. (2014). Genetic improvement of Israeli blue (Jordan) tilapia, Oreochromis aureus (Steindachner), through selective breeding for harvest weight. Aquaculture Research, 45(3):546-557.
- Zhang, Z. H., Chen, J., Li, L., Tao, M., Zhang, C., Qin, Q. B., Xiao, J., Liu, Y., & Liu, S. J. (2014). Research advances in animal distant hybridization. Science China. Life Sciences, 57(9):889-902.
- Zhong, H., Zhang, X., Xu, Q., Yan, J., Han, Z., Zheng, H., Xiao, J., Tang, Z., Wang, F., Luo, Y., & Zhou, Y. (2019). Nonadditive and asymmetric allelic expression of growth hormone in hybrid tilapia. Frontiers in Genetics, 10(1):1-11.
- Zhou, Y., Zhang, X., Xu, Q., Yan, J., Yu, F., Wang, F., Xiao, J., Luo, Y., & Zhong, H. (2019). Nonadditive and allele-specific expression of insulin-like growth factor 1 in Nile tilapia (Oreochromis niloticus, ♀) × blue tilapia (O. aureus, ♂) hybrids. Comparative Biochemistry and Physiology Part - B: Biochemistry and Molecular Biology, 232(6):93-100.
References
Abdel-Tawwab, M., Ahmad, M. H., Khattab, Y. A. E., & Shalaby, A. M. E. (2010). Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture, 298(3–4):267-274.
Abwao, J., Kyule, D., Junga, J. O., Barasa, J. E., & Sigana, D. A. (2023). On‐farm growth performance of different strains of tilapia, Oreochromis niloticus reared in earthen ponds. Aquaculture, Fish and Fisheries, 3(3):247-255.
Akian, D. D., Yao, K., Clota, F., Lozano, P., Baroiller, J. F., Chatain, B., & Bégout, M. L. (2017). Reproductive behaviour of two tilapia species (Oreochromis niloticus, Linné, 1758; Sarotherodon melanotheron, Rüppel, 1852) in freshwater intra and interspecific pairing context. Applied Animal Behaviour Science, 193(8):104-113.
Almeida, D. B., da Costa, M. A. P., Bassini, L. N., Calabuig, C. I. P., Moreira, C. G. A., Rodrigues, M. D. N., Pérez, H. J., Tavares, R. A., Varela, A. S., & Moreira, H. L. M. (2013). Reproductive performance in female strains of Nile tilapia, Oreochromis niloticus. Aquaculture International, 21(6):1291-1300.
Altinok, I., Ozturk, R. C., Capkin, E., & Kalayci, G. (2020). Experimental crossbreeding reveals variation in growth among brown trout (Salmo trutta) strains and their reciprocal crossbreeds. Aquaculture, 521(8):1-9.
Arifin, O. Z., Imron, Aseppendi, Hendri, A., Muslim, N., & Yani, A. (2017). Intraspecific hybridization between two populations of Galunggung giant gourami (Osphronemus goramy Lacepede, 1801). Jurnal Riset Akuakultur, 12(4):315-323.
Bartie, K. L., Taslima, K., Bekaert, M., Wehner, S., Syaifudin, M., Taggart, J. B., de Verdal, H., Rosario, W., Muyalde, N., Benzie, J. A. H., McAndrew, B. J., & Penman, D. J. (2020). Species composition in the Molobicus hybrid tilapia strain. Aquaculture, 526(13):1-7.
Basavaraja, N., & Raghavendra, C. H. (2017). Hormonal sex reversal in red tilapia (Oreochromis niloticus and Oreochromis mossambicus) and inheritance of body colour in O. mossambicus and red tilapia: Implications for commercial farming. Aquaculture International, 25(3):1317-1331.
Bentsen, H. B., Eknath, A. E., Palada-de Vera, M. S., Danting, J. C., Bolivar, H. L., Reyes, R. A., Dionisio, E. E., Longalong, F. M., Circa, A. V., Tayamen, M. M., & Gjerde, B. (1998). Genetic improvement of farmed tilapias: Growth performance in a complete diallel cross experiment with eight strains of Oreochromis niloticus. Aquaculture, 160(1–2):145-173.
Boyd, C. E. (2015). Water quality: An introduction. 2nd Edition. Springer.
Bradbeer, S. J., Harrington, J., Watson, H., Warraich, A., Shechonge, A., Smith, A., Tamatamah, R., Ngatunga, B. P., Turner, G. F., & Genner, M. J. (2019). Limited hybridization between introduced and critically endangered indigenous tilapia fishes in northern Tanzania. Hydrobiologia, 832(1):257-268.
Cao, J., Yang, N., Liu, Z., Lu, M., Gao, F., Ke, X., Wang, M., & Yi, M. (2021). Distant hybridization and gynogenesis between Nile tilapia Oreochromis niloticus and Jaguar cichlid Parachromis managuensis. Animal Reproduction Science, 232(9):1-12.
Chen, J., Fan, Z., Tan, D., Jiang, D., & Wang, D. (2018). A review of genetic advances related to sex control and manipulation in tilapia. Journal of the World Aquaculture Society, 49(2):277-291.
Chen, S., Tian, Y., Li, Z., Liu, Y., Li, Z., Duan, P., Li, L., Wang, X., Wang, L., He, X., Zhao, X., Li, W., & Wang, Q. (2023). Heterosis in growth and low temperature tolerance in Jinhu grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂). Aquaculture, 562(1):1-13.
Correia, D., Escarcega-Miranda, B., Barreto-Curiel, F., Mata-Sotres, J., del Rio-Zaragoza, O., Viana, M. T., & Rombenso, A. N. (2019). Growth performance and body composition of hybrid red tilapia (Oreochromis mossambicus × O. aureus) fed with different protein levels raised in saltwater. Latin American Journal of Aquatic Research, 47(5):853-859.
Coward, K., & Bromage, N. R. (2000). Reproductive physiology of female tilapia broodstock. Reviews in Fish Biology and Fisheries, 10(1):1-25.
Crespel, A., Audet, C., Bernatchez, L., & Garant, D. (2012). Effects of rearing environment and strain combination on heterosis in brook trout. North American Journal of Aquaculture, 74(2):188-198.
Cruz, P., & Ibarra, A. M. (1997). Larval growth and survival of two catarina scallop (Argopecten circularis, Sowerby, 1835) populations and their reciprocal crosses. Journal of Experimental Marine Biology and Ecology, 212(1):95-110.
Dai, P., Wang, H., Xiao, G., & Liu, B. (2014). Combining ability and heterosis analysis over two environments in a diallel cross of three families of the clam Meretrix meretrix. Acta Oceanologica Sinica, 33(10):37-42.
De Donato, M., Manrique, R., Ramirez, R., Mayer, L., & Howell, C. (2005). Mass selection and inbreeding effects on a cultivated strain of Penaeus (Litopenaeus) vannamei in Venezuela. Aquaculture, 247(1–4):159-167.
De Verdal, H., Rosario, W., Vandeputte, M., Muyalde, N., Morissens, P., Baroiller, J. F., & Chevassus, B. (2014). Response to selection for growth in an interspecific hybrid between Oreochromis mossambicus and O. niloticus in two distinct environments. Aquaculture, 430(8):159-165.
Delomas, T. A., Gomelsky, B., Vu, N., Campbell, M. R., & Novelo, N. D. (2019). Single-nucleotide polymorphism discovery and genetic variation in YY-male and mixed-sex strains of Nile tilapia available in the United States. North American Journal of Aquaculture, 81(3):183-188.
Desprez, D., Briand, C., Hoareau, M. C., Mélard, C., Bosc, P., & Baroiller, J. F. (2006). Study of sex ratio in progeny of a complex Oreochromis hybrid, the Florida red tilapia. Aquaculture, 251(2–4):231-237.
El-Araby, D. A., Amer, S. A., & Khalil, A. A. (2020). Effect of different feeding regimes on the growth performance, antioxidant activity, and health of Nile tilapia, Oreochromis niloticus. Aquaculture, 528(15):1-10.
El-Sayed, A.-F. M. (2020a). The role of tilapia culture in rural development. Tilapia Culture, 275-295.
El-Sayed, A.-F. M. (2020b). Environmental requirements. Tilapia Culture, 47-67.
Falconer, D. S., & Mackay, T. F. C. (1996). Introduction to quantitative genetics. 4th Ed. England: Longman.
Fehr, W. R. (1991). Principles of cultivar development, theory and technique. New York: Macmillan Publishing Company.
Goddard, S. (1996). Feeds in intensive aquaculture. In S. Goddard. Feed management in intensive aquaculture. (pp. 1-22). Springer.
Gomelsky, B. (2011). Fish genetics: Theory and practice. Saarbrücken, Germany: VDM Verlag Dr. Mueller GmbH & Co.
Granier, S., Audet, C., & Bernatchez, L. (2011). Heterosis and outbreeding depression between strains of young-of-the-year brook trout (Salvelinus fontinalis). Canadian Journal of Zoology, 89(3):190-198.
Githukia, C. M., Ogello, E. O., Kembenya, E. M., Achieng, A. O., Obiero, K. O., & Munguti, J. M. (2015). Comparative growth performance of male monosex and mixed sex nile tilapia (Oreochromis niloticus l.) reared in earthen ponds. Ribarstvo, Croatian Journal of Fisheries, 73(1):20-25.
Hallauer, A. R., Carena, M. J., & Filho, J. B. M. (2010). Heterosis. In: Quantitative Genetics in Maize Breeding. Part of the book series: Handbook of Plant Breeding. Vol 6. (pp. 477-529). New York: Springer.
Hamzah, A., Nguyen, N. H., Mekkawy, W., Khaw, H. L., Yee, H. Y., Abu Bakar, K. R., Ponzoni, R. W., & Mohd Nor, S. A. (2016). Genetic parameters and correlated responses in female reproductive traits in the GIFT strain. Aquaculture Research, 47(5):1488-1498.
Hasan, M & Soto, D. (2017). Improving feed conversion ratio and its impact on reducing greenhouse gas. Rome: Food and Agriculture Organization of The United Nations.
Herawati,V. E., Susilo, A., Pinandoyo, Hutabarat, J., Sugianto, D. N., Wirasatriya, A., & Karnaradjasa, O. (2019). Optimization of fish meal substitution with maggot meal (Hermetia Illucens) for growth and feed utilization efficiency of juvenile Litopenaeus vannamei. Asian Journal of Microbiology, Biotechnology & Environmental Sciences, 21(2):284-297.
Hernández-Gurrola, J. A., Naranjo-Páramo, J., Vargas-Mendieta, M., Cruz-Hernández, P., Villarreal-García, A., Mora-Castrejón, G., & Villarreal-Colmenares, H. (2020). Effect of crossbreeding three divergent populations on the juvenile production and rearing performance of the redclaw crayfish Cherax quadricarinatus. Aquaculture, 527(14):1-10.
Hovick, S. M., & Whitney, K. D. (2014). Hybridisation is associated with increased fecundity and size in invasive taxa: Meta-analytic support for the hybridisation-invasion hypothesis. Ecology Letters, 17(11):1464-1477.
Huxel, G. R. (1999). Rapid displacement of native species by invasive species: Effects of hybridization. Biological Conservation, 89(2):143-152.
Jiang, G., Li, Q., Xu, C., Liu, S., Kong, L., & Yu, H. (2021). Reciprocal hybrids derived from Crassostrea gigas and C. angulata exhibit high heterosis in growth, survival and thermotolerance in northern China. Aquaculture, 545(16):1-9.
Jiang, G., Li, Q., & Xu, C. (2022). Growth, survival and gonad development of two new types of reciprocal triploid hybrids between Crassostrea gigas and C. angulata. Aquaculture, 559(15):1-11.
Joshi, R., Woolliams, J. A., Meuwissen, T. H. E., & Gjøen, H. M. (2018). Maternal, dominance and additive genetic effects in Nile tilapia; influence on growth, fillet yield and body size traits. Heredity, 120(5):452-462.
Khaw, H. L., Ponzoni, R. W., Yee, H. Y., bin Aziz, M. A., & Bijma, P. (2016). Genetic and non-genetic indirect effects for harvest weight in the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture, 450(1):154-161.
Kong, L., Song, S., & Li, Q. (2017). The effect of interstrain hybridization on the production performance in the Pacific oyster Crassostrea gigas. Aquaculture, 472(7):44-49.
Koolboon, U., Koonawootrittriron, S., Kamolrat, W., & Na-Nakorn, U. (2014). Effects of parental strains and heterosis of the hybrid between Clarias macrocephalus and Clarias gariepinus. Aquaculture, 424–425(5):131-139.
Krasnovyd, V., Vetešník, L., & Šimková, A. (2020). Distribution of host-specific parasites in hybrids of phylogenetically related fish: The effects of genotype frequency and maternal ancestry? Parasites and Vectors, 13(1):1-11.
In, V. Van, Sang, V. Van, O’Connor, W., Van, P. T., Dove, M., Knibb, W., & Nguyen, N. H. (2017). Are strain genetic effect and heterosis expression altered with culture system and rearing environment in the Portuguese oyster (Crassostrea angulata)? Aquaculture Research, 48(8):4058-4069.
Lago, A. de A., Rezende, T. T., Dias, M. A. D., Freitas, R. T. F. de, & Hilsdorf, A. W. S. (2017). The development of genetically improved red tilapia lines through the backcross breeding of two Oreochromis niloticus strains. Aquaculture, 472(8):17-22.
Lal, M. M., Waqairatu, S. S., Zenger, K. R., Nayfa, M. G., Pickering, T. D., Singh, A., & Southgate, P. C. (2021). The GIFT that keeps on giving? A genetic audit of the Fijian genetically improved farmed tilapia (GIFT) broodstock nucleus 20 years after introduction. Aquaculture, 537(8):1-18.
Li, Z., Coffey, L., Garfin, J., Miller, N. D., White, M. R., Spalding, E. P., De Leon, N., Kaeppler, S. M., Schnable, P. S., Springer, N. M., & Hirsch, C. N. (2018). Genotype-by-environment interactions affecting heterosis in maize. PLoS One, 13(1):1-16.
Liang, Y., Xu, C., & Li, Q. (2023). Growth, survival and color segregation of F2 hybrids between selected “Haida no.1” and orange-shell lines of the Pacific oyster. Aquaculture, 574(14):1-8.
Liang, Y., Xu, C., & Li, Q. (2023). Heterosis and genetic diversity of intraspecific hybrids crosses between two selected lines of the Pacific oyster Crassostrea gigas. Aquaculture, 569(9):1-9.
Lind, C. E., Ponzoni, R. W., Nguyen, N. H., & Khaw, H. L. (2012). Selective breeding in fish and conservation of genetic resources for aquaculture. Reproduction in Domestic Animals, 47(4):255–263.
Liu, S. J. (2010). Distant hybridization leads to different ploidy fishes. Science China Life Sciences, 53(4):416-425.
Lozano, C. A., Gjerde, B., Ødegård, J., Rye, M., & Dinh, T. (2014). Heritability estimates for male proportion in hybrids between Nile tilapia females (Oreochromis niloticus ) and blue tilapia males (Oreochromis aureus). Aquaculture, 430(8):66-73.
Lu, B., Liang, G., Xu, M., Wang, C., Tan, D., Tao, W., Sun, L., & Wang, D. (2022). Production of all male amelanotic red tilapia by combining MAS-GMT and tyrb mutation. Aquaculture, 546(1):1-11.
Maluwa, A. O., & Gjerde, B. (2006a). Genetic evaluation of four strains of Oreochromis shiranus for harvest body weight in a diallel cross. Aquaculture, 259(1–4):28-37.
Maluwa, A. O., & Gjerde, B. (2006b). Estimates of the strain additive, maternal and heterosis genetic effects for harvest body weight of an F2 generation of Oreochromis shiranus. Aquaculture, 259(1–4):38-46.
Marengoni, N. G., Onoue, Y., & Oyama, T. (1998). Offspring growth in a diallel crossbreeding with three strains of Nile tilapia Oreochromis niloticus. Journal of the World Aquaculture Society, 29(1):114-119.
MMAF. (2022). Marine and fisheries in figures 2022. Jakarta: The Center for Data, Statistics and Information. Ministry of Marine Affairs and Fisheries of the Republic of Indonesia.
Mashaii, N., Rajabipour, F., Mohammadi, M., Sarsangi, H., Bitaraf, A., Hossein-Zadeh, H., & Sharif-Rohani, M. (2016). Reproduction of Nile tilapia, Oreochromis niloticus in brackish water. Journal of Applied Aquaculture, 28(1):1-8.
Mbiru, M., & Mchele, S. (2015). Comparative performance of mixed-sex and hormonal- sex-reversed Nile tilapia Oreochromis niloticus. Aquaculture International, 24(2):557-566.
Mengistu, S. B., Mulder, H. A., Benzie, J. A. H., Khaw, H. L., Megens, H. J., Trinh, T. Q., & Komen, H. (2020). Genotype by environment interaction between aerated and non-aerated ponds and the impact of aeration on genetic parameters in Nile tilapia (Oreochromis niloticus). Aquaculture, 529(16):1-10.
Moses, M., Chauka, L. J., de Koning, D. J., Palaiokostas, C., & Mtolera, M. S. P. (2021). Growth performance of five different strains of Nile tilapia (Oreochromis niloticus) introduced to Tanzania reared in fresh and brackish waters. Scientific Reports, 11(1):1-12.
Mtaki, K., Limbu, S. M., Mmochi, A. J., & Mtolera, M. S. P. (2022). Hybrids production as a potential method to control prolific breeding in tilapia and adaptation to aquaculture climate-induced drought. Aquaculture and Fisheries, 7(6):647-652.
NRC [National Research Council]. (1983). Nutrient requirements of warmwater fishes. Sub Committee on Warmwater Fish Nutrition. Committee on Animal Nutrition Board on Agriculture and Renewable Resources. Washington DC: National Academy Science.
Neira, R., García, X., Paul, J., Filp, M., Manuel, J., & María, A. (2016). Evaluation of the growth and carcass quality of diallel crosses of four strains of Nile tilapia ( Oerochromis niloticus ). Aquaculture, 451(12):213-222.
Novelo, N. D., Gomelsky, B., Coyle, S. D., & Kramer, A. G. (2021). Evaluation of growth, sex (male proportion; sexual dimorphism), and color segregation in four cross combinations of different strains of XX female and YY male Nile Tilapia. Journal of the World Aquaculture Society, 52(2):445-456.
Nugroho, E., Rustadi, Priyanto, D., Sulistyo, H., Susila, Sunaryo & Wasito, B. (2014). Lost of genetic variation of genetically improved strain of “Cangkringan” red tilapia monitored in F-4 detected by DNA Marker. Jurnal Riset Akuakultur, 9(1):25-30.
Nzohabonayo, E., Manyala, J., Kang’ombe, J., & Kassam, D. (2021). Effect of hybridization on reproductive performance of Oreochromis karongae, Oreochromis shiranus and Oreochromis mossambicus. Aquaculture Research, 52(1):302-308.
Omasaki, S. K., Charo-karisa, H., Kahi, A. K., & Komen, H. (2016). Genotype by environment interaction for harvest weight, growth rate and shape between monosex and mixed sex Nile tilapia (Oreochromis niloticus). Aquaculture, 458(9):75-81.
Osure, G. O., & Phelps, R. P. (2006). Evaluation of reproductive performance and early growth of four strains of Nile tilapia (Oreochromis niloticus L) with different histories of domestication. Aquaculture, 253(1–4):485-494.
Piwpong, N., Chiayvareesajja, J., & Chiayvareesajja, S. (2017). Growth and survival of a diallel cross for five strains of climbing perch (Anabas testudineus Bloch, 1792) in Thailand. Agriculture and Natural Resources, 50(5):351-356.
Pongthana, N., Hong, N., & Ponzoni, R. W. (2010). Comparative performance of four red tilapia strains and their crosses in fresh and saline water environments. Aquaculture, 308(12):S109-S114.
Ponzoni, R. W., Nguyen, N. H., Khaw, H. L., Hamzah, A., Bakar, K. R. A., & Yee, H. Y. (2011). Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the World Fish Center with the GIFT strain. Reviews in Aquaculture, 3(1):27-41.
Poolsawat, L., Yu, Y., Li, X., Zhen, X., Yao, W., Wang, P., Luo, C., & Leng, X. (2022). Efficacy of phytogenic extracts on growth performance and health of tilapia (Oreochromis niloticus × O. aureus). Aquaculture and Fisheries, 7(4):411-419.
Qin, Y., Li, X., Liao, Q., Li, J., Ma, H., Mo, R., Zhang, Y., & Yu, Z. (2021). Comparative study on the growth, survival, gonad development and trait segregation of F2 hybrids and their grandparent species (Crassostrea ariakensis and C. hongkongensis). Aquaculture, 541(12):1-9.
Radona, D., Subagja, J., Otong, D., Arifin, Z., Penelitian, B., Budidaya, P., & Tawar, A. (2016). Performance of reproductive aspects of broodstock and growth of seed of Tor soro, Tor douronensis, and their reciprocal crosses. Jurnal Riset Akuakultur, 10(3):335-343.
Rahman, M. A., Arshad, A., Marimuthu, K., Ara, R., & Amin, S. M. N. (2013). Inter-specific hybridization and its potential for aquaculture of fin fishes. Asian Journal of Animal and Veterinary Advances. 8(2):139-153.
Rapatsa, M. M., & Moyo, N. A. G. (2017). Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquaculture Reports, 5(1):18-26.
Rasidi., Nugroho, E., Radona, D., Emmawatie, L., & Ardi, I. (2015). Heterosis value and growth performance of four populations of tilapia (Oreochromis niloticus) resulting from reciprocal breeding. Prosiding Forum Teknologi Akuakultur 2015: 183–190.
Ricker, W. E. (1979). Growth rates and models. Fish Physiology, 8(1):677-743.
Ridha, M. T. (2012). Preliminary observations on growth and survival of Oreochromis spilurus x GIFT Oreochromis niloticus F 1 reciprocal hybrids in fresh and seawater. Aquaculture Research, 45(3):1-9.
Robisalmi, A., Gunadi, B., & Setyawan, P. (2020). Evaluation of Growth Performance and Heterosis of hybridization between female nile tilapia (Oreochromis niloticus) with male blue tilapia (Oreochromis aureus) F2 on Hipersalinity Brakish water Pond. Berita Biologi, 19(1):1-11.
Robisalmi, A., Alipin, K., & Gunadi, B. (2021). Growth performance and microstructure in the muscle fiber of red tilapia ( Oreochromis spp.) under different cycles of fasting and refeeding. AACL Bioflux, 14(3):1498-1512.
Rossato, K. A., Mauerwerk, M. T., Balen, R. E., Zadinelo, I. V., & da Silva, L. C. R. (2022). Interaction between weight and strain of Nile tilapia broodstocks in relation to growth and reproductive performance. Aquaculture, 559(15):1-5.
Russell, D. J., Thuesen, P. A., & Thomson, F. E. (2012). Reproductive strategies of two invasive tilapia species Oreochromis mossambicus and Tilapia mariae in northern Australia. Journal of Fish Biology, 80(6):2176-2197.
Saleky, D., Sianturi, R., Dailami, M., & Kusuma, A. B. (2021). Molecular study of Oreochromis spp. from Merauke Waterland-Papua, based on mitochondrial DNA cytochrome oxidase subunit I fragment gene. Jurnal Perikanan Universitas Gadjah Mada, 23(1):37-43.
Sarker, B. S., Sabuz, M. S. R., Azom, M. G., Easmin, N., Ali, A., Alam, M. S., & Islam, M. S. (2023). Genotype responsive growth performance and salinity tolerance of tilapia hybrid (Oreochromis niloticus × O. mossambicus). Aquaculture, 575(15):1-9.
Sarmento, N. L. A. F., Martins, E. F. F., Costa, D. C., Mattioli, C. C., da Costa Julio, G. S., Figueiredo, L. G., Luz, M. R., & Luz, R. K. (2018). Reproductive efficiency and egg and larvae quality of Nile tilapia fed different levels of vitamin C. Aquaculture, 482(1):96-102.
Shivaramu, S., Vuong, D. T., Havelka, M., Šachlová, H., Lebeda, I., Kašpar., V., & Flajšhans, M. (2019). Influence of interspecific hybridization on fitness-related traits in Siberian sturgeon and Russian sturgeon. Czech Journal of Animal Science, 64(2):78-88.
Silva, A. C. F., Filho, R. A. C. C., Ventura, A. S., Nunes, A. L., Laice, L. M., Ribeiro, R. P., Oliveira, C. A. L., Almeida, L. C., Barbosa, P. T. L., & Povh, J. A. (2020). Reproductive traits in different Nile tilapia genetic groups [Caracteristicas reprodutivas em diferentes grupos geneticos de tilápia do Nilo]. Arquivo Brasileiro de Medicina Veterinári e Zootecnia, 72(5):1797-1804.
Silva, A. C. C., Corrêa Filho, R. A. C., Fornari, D. C., Abreu, J. S. de, Bignardi, A. B., Severino, M. de F. G., Amorim, L. F. dos S., Albuquerque, L. V., Carneiro, I. L., & Povh, J. A. (2022). Production of tambaqui and of the tambatinga and tambacu hybrids: Performance, morphometric traits, and body yield. Aquaculture, 554(10):1-7.
Šimková, A., Janáč, M., Hyršl, P., Krasnovyd, V., & Vetešník, L. (2021). Vigour-related traits and immunity in hybrids of evolutionary divergent cyprinoid species: advantages of hybrid heterosis? Journal of Fish Biology, 98(4):1155-1171.
Snake, M., Maluwa, A., Zidana, H., Chigwechokha, P., & Simwaka, M. (2020). Production of a predominantly male tilapia progeny using two Malawian tilapias, Oreochromis shiranus and Oreochromis karongae. Aquaculture Reports, 16(1):1-5.
Söderquist, L., Broberg, A., Rosenberg, V., & Sletvold, N. (2020). Predicting heterosis and inbreeding depression from population size and density to inform management efforts. Journal of Applied Ecology, 57(8):1459-1468.
Subekti, S., Arief, M., & Yudha, G. C. P. (2016). Silage substitution chemically solid waste surimi fish swanggi (Priacanthus macracanthus) on to retention protein fish meal and retention fat tilapia (Oreochromis niloticus). Jurnal Ilmiah Perikanan dan Kelautan, 8(2):77-83.
Sun, C., Dong, J., Li, W., Tian, Y., Hu, J., & Ye, X. (2022). Response to four generations of selection for growth performance traits in mandarin fish (Siniperca chuatsi). Aquaculture, 548(3):1-6.
Tave, D. (1995). Selective breeding programmes for medium-sized fish farms. In FAO. Fisheries Technical Paper. Vol. 352.
Tave, D., Jayaprakas, V., & Smitherman O. R. (1990). Effects of Intraspecific Hybridization in. Journal of the World Aquaculture Society, 21(3):201-204.
Thoa, N. P., Ninh, N. H., Hoa, N. T., Knibb, W., Diep, N. H., & Nguyen, N.H. (2016). Additive genetic and heterotic effects in a 4 x 4 complete diallel cross-population of Nile tilapia (Oreochromis niloticus, Linnaeus, 1758) reared in different water temperature environments in Northern Vietnam. Aquacultre Research, 47(3):708-720.
Thoa, N. P., Hamzah, A., & Nguyen, N. H. (2017). Genetic variation and correlated changes in reproductive performance of a red tilapia line selected for improved growth over three generations. Animal Reproduction Science, 184(9):94-101.
Thodesen Da-Yong Ma, J., Rye, M., Wang, Y. X., Yang, K. S., Bentsen, H. B., & Gjedrem, T. (2011). Genetic improvement of tilapias in China: Genetic parameters and selection responses in growth of Nile tilapia (Oreochromis niloticus) after six generations of multi-trait selection for growth and fillet yield. Aquaculture, 322–323(18):51-64.
Thongprajukaew, K., Kovitvadhi, S., Kovitvadhi, U., & Preprame, P. (2017). Effects of feeding frequency on growth performance and digestive enzyme activity of sex-reversed Nile tilapia, Oreochromis niloticus (Linnaeus, 1758). Agriculture and Natural Resources, 51(4):292-298.
Todesco, M., Pascual, M. A., Owens, G. L., Ostevik, K. L., Moyers, B. T., Hübner, S., Heredia, S. M., Hahn, M. A., Caseys, C., Bock, D. G., & Rieseberg, L. H. (2016). Hybridization and extinction. Evolutionary Applications, 9(7):892-908.
Trinh, T. Q., Agyakwah, S. K., Khaw, H. L., Benzie, J. A. H., & Attipoe, F. K. Y. (2021). Performance evaluation of Nile tilapia (Oreochromis niloticus) improved strains in Ghana. Aquaculture, 530(1):1-6.
Trong, T. Q., Van Arendonk, J. A. M., & Komen, H. (2013). Genetic parameters for reproductive traits in female Nile tilapia (Oreochromis niloticus): II. Fecundity and fertility. Aquaculture, 416–417(16):72-77.
Tuan Harith, Z., Mohd Sukri, S., Remlee, N. F. S., Mohd Sabir, F. N., & Zakaria, N. N. A. (2022). Effects of dietary astaxanthin enrichment on enhancing the colour and growth of red tilapia, Oreochromis sp. Aquaculture and Fisheries, 9(1):52-56.
Valentin, F. N., do Nascimento, N. F., da Silva, R. C., Tsuji, E. A., Paes, M. C. F., Koberstein, T. C. R. D., & Nakaghi, L. S. O. (2015). Maternal age influences on reproductive rates in Nile tilapia (Oreochromis niloticus). Revista Brasileira de Zootecnia, 44(4):161-163.
Wang, C. H., Li, S. F., Liu, Z. G., Xiang, S. P., Wang, J., Pang, Z. Y., & Duan, J. P. (2006). Developmental quantitative genetic analysis of body weight and morphological traits in red common carp, Cyprinus carpio L. Aquaculture, 251(2–4):219–230.
Wang, C., & Li, Z. (2010). Improvement in production traits by mass spawning type crossbreeding in bay scallops. Aquaculture, 299(1–4):51-56.
Wang, S., Tang, C., Tao, M., Qin, Q., Zhang, C., & Luo, K. (2019). Establishment and application of distant hybridization technology in fish. Science China Life Sciences, 62(1):22-45.
Whitlock, M. C., Ingvarsson, P. K., & Hatfield, T. (2000). Local drift load and the heterosis of interconnected populations. Heredity, 84(4):452-457.
Workakegn, B. K. (2019). Establishment of base population for long-term genetic improvement of Nile tilapia in Ethiopia. PhD dissertation. Norway: NMBU.
Xiao, Q., Shen, Y., Gan, Y., Wang, Y., Zhang, J., Huang, Z., You, W., Luo, X., & Ke, C. (2022). Three-way cross hybrid abalone exhibit heterosis in growth performance, thermal tolerance, and hypoxia tolerance. Aquaculture, 555(11):1-9.
Xing, Q., Yang, Z., Zhu, X., Liu, J., Huang, X., Hu, J., & Bao, Z. (2022). Interspecific hybridization between Patinopecten yessoensis (♀) and (♂) with heterosis in growth and temperature tolerance. Aquaculture, 547(2):1-14.
Xu, K., Duan, W., Xiao, J., Tao, M., Zhang, C., Liu, Y., & Liu, S. J. (2015). Development and application of biological technologies in fish genetic breeding. Science China Life Sciences, 58(2):187-201.
Yamasaki, L. S., Iwai, T., Klinger-Bowen, R. E. C., Weese, D. A., Fowler, C. E., Yacoub, J. L., & Wong, M. A. (2022). Identification of Nile tilapia (Oreochromis niloticus) and its hybrids in natural environments in Hawaii. Aquaculture, 550(6):1-5.
Yan, L., Su, J., Wang, Z., Zhang, Y., Yan, X., & Yu, R. (2018). Growth performance and biochemical composition of the oysters Crassostrea sikamea, Crassostrea angulata and their hybrids in Southern China. Aquaculture Research, 49(2):1020-1028.
Yoshida, G. M., de Oliveira, C. A. L., Kunita, N. M., Rizzato, G. S., & Ribeiro, R. P. (2015). Reproduction performance of female Nile tilapia under different environments and age classes. Acta Scientiarum - Animal Sciences, 37(3):221-226.
Yu, D., Gu, X., Zhang, S., Dong, S., Miao, H., Gebretsadik, K., & Bo, K. (2021). Molecular basis of heterosis and related breeding strategies reveal its importance in vegetable breeding. Horticulture Research, 8(1):1-17.
Zak, T., Deshev, R., Benet-Perlberg, A., Naor, A., Magen, I., Shapira, Y., Ponzoni, R. W., & Hulata, G. (2014). Genetic improvement of Israeli blue (Jordan) tilapia, Oreochromis aureus (Steindachner), through selective breeding for harvest weight. Aquaculture Research, 45(3):546-557.
Zhang, Z. H., Chen, J., Li, L., Tao, M., Zhang, C., Qin, Q. B., Xiao, J., Liu, Y., & Liu, S. J. (2014). Research advances in animal distant hybridization. Science China. Life Sciences, 57(9):889-902.
Zhong, H., Zhang, X., Xu, Q., Yan, J., Han, Z., Zheng, H., Xiao, J., Tang, Z., Wang, F., Luo, Y., & Zhou, Y. (2019). Nonadditive and asymmetric allelic expression of growth hormone in hybrid tilapia. Frontiers in Genetics, 10(1):1-11.
Zhou, Y., Zhang, X., Xu, Q., Yan, J., Yu, F., Wang, F., Xiao, J., Luo, Y., & Zhong, H. (2019). Nonadditive and allele-specific expression of insulin-like growth factor 1 in Nile tilapia (Oreochromis niloticus, ♀) × blue tilapia (O. aureus, ♂) hybrids. Comparative Biochemistry and Physiology Part - B: Biochemistry and Molecular Biology, 232(6):93-100.