FORMULASI DAN KARAKTERISASI SEDIAAN NANOEMULSI VITAMIN A <br><i>[Formulation and Characterization of Vitamin A Nanoemulsion]</i>
Downloads
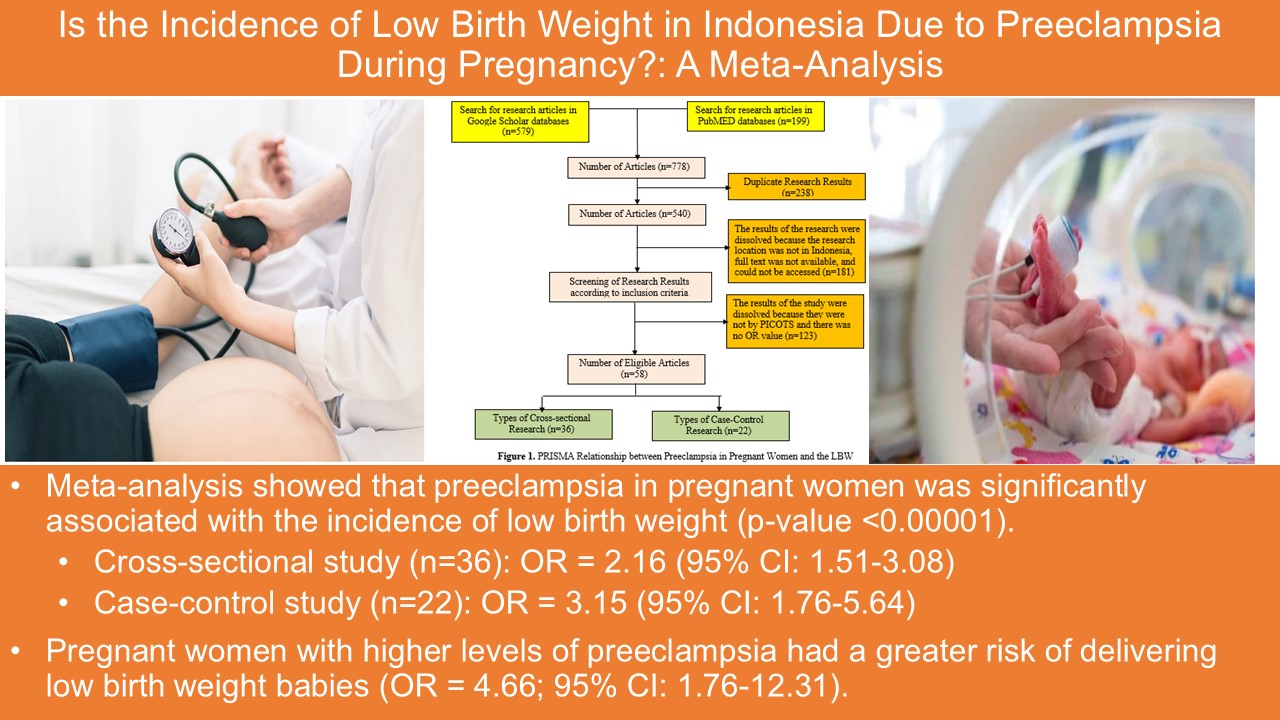
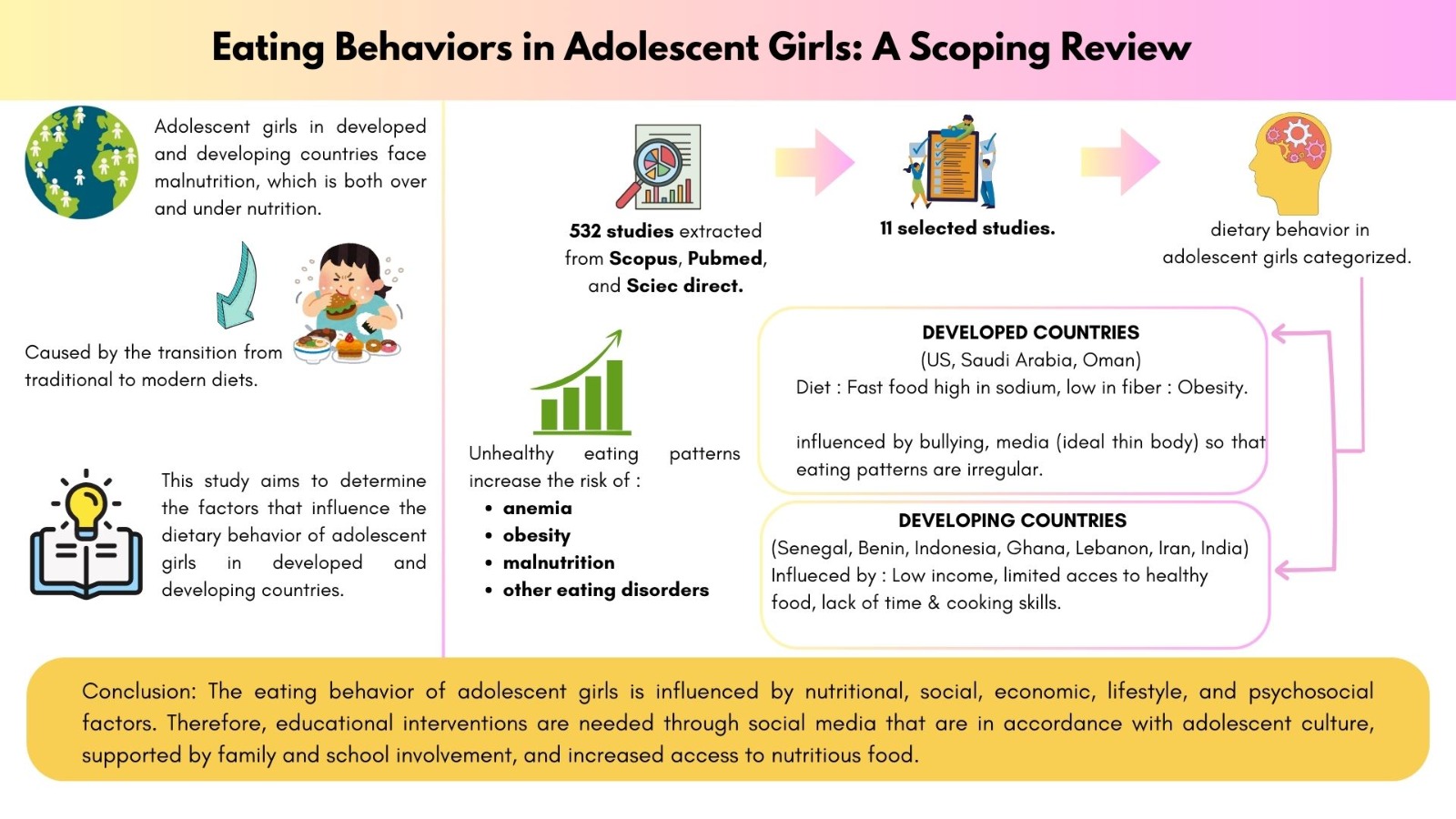
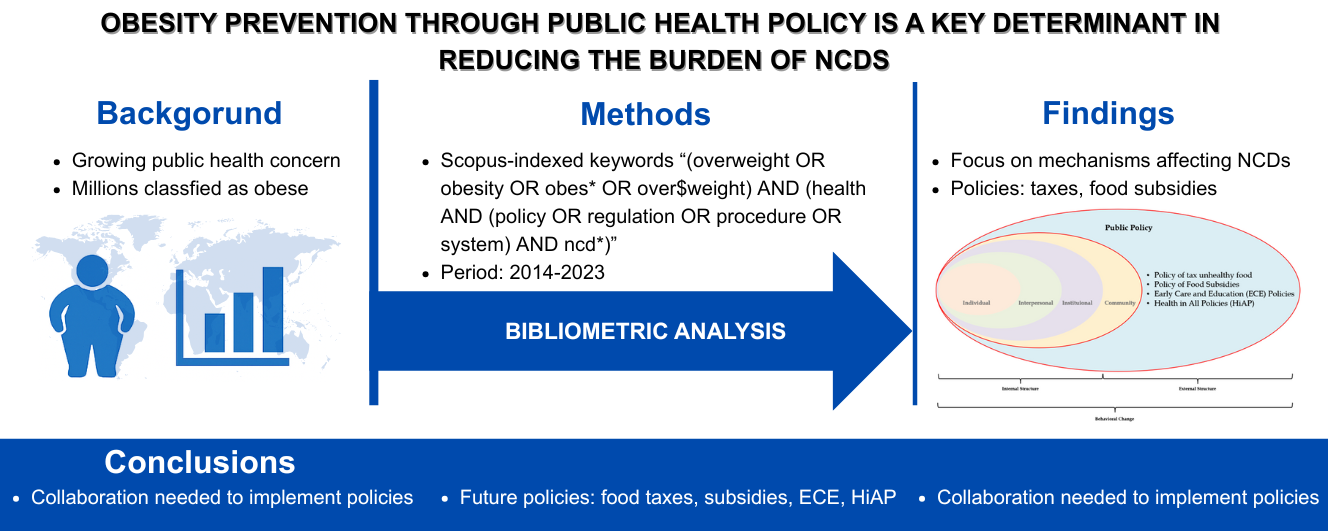
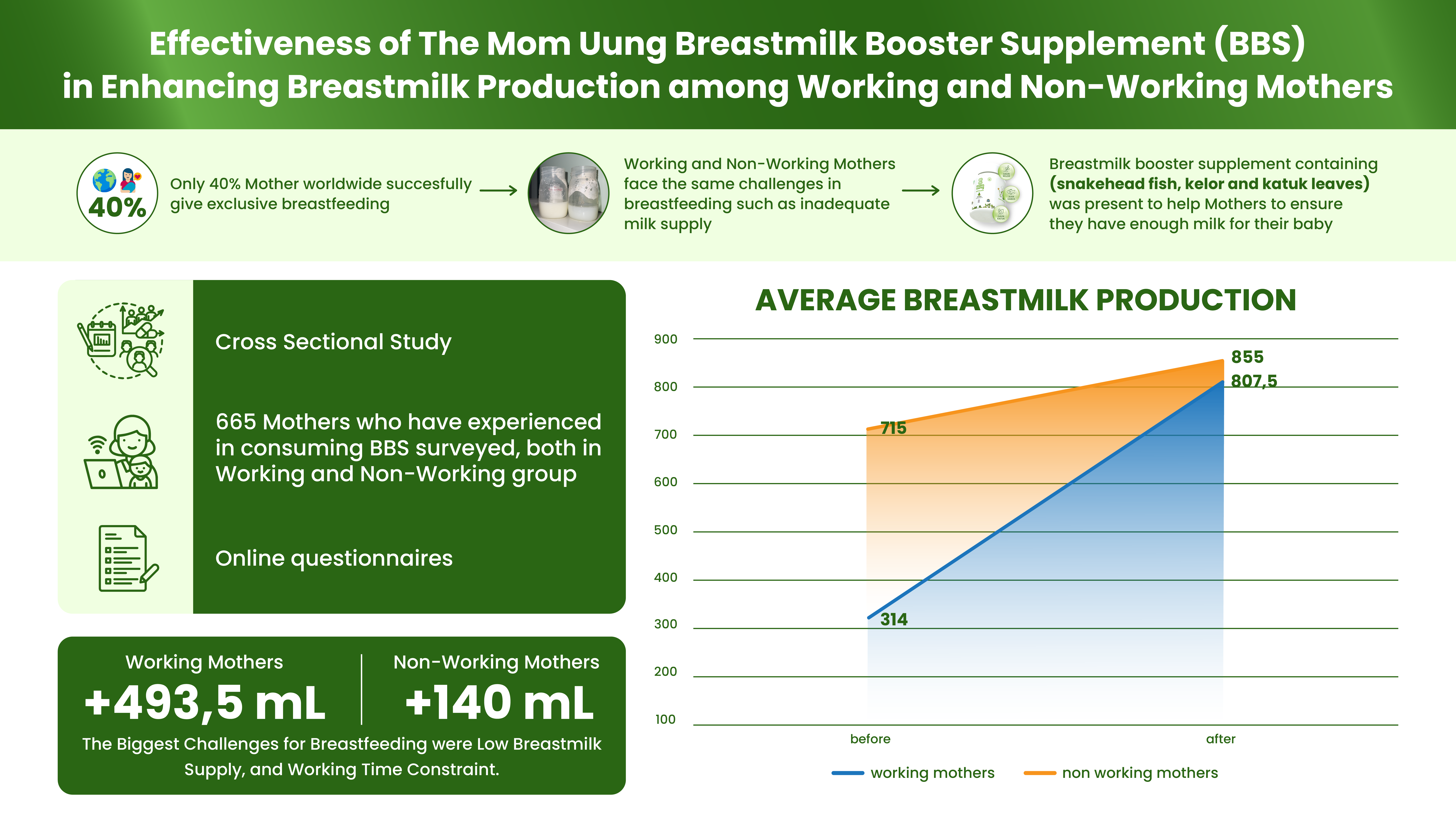
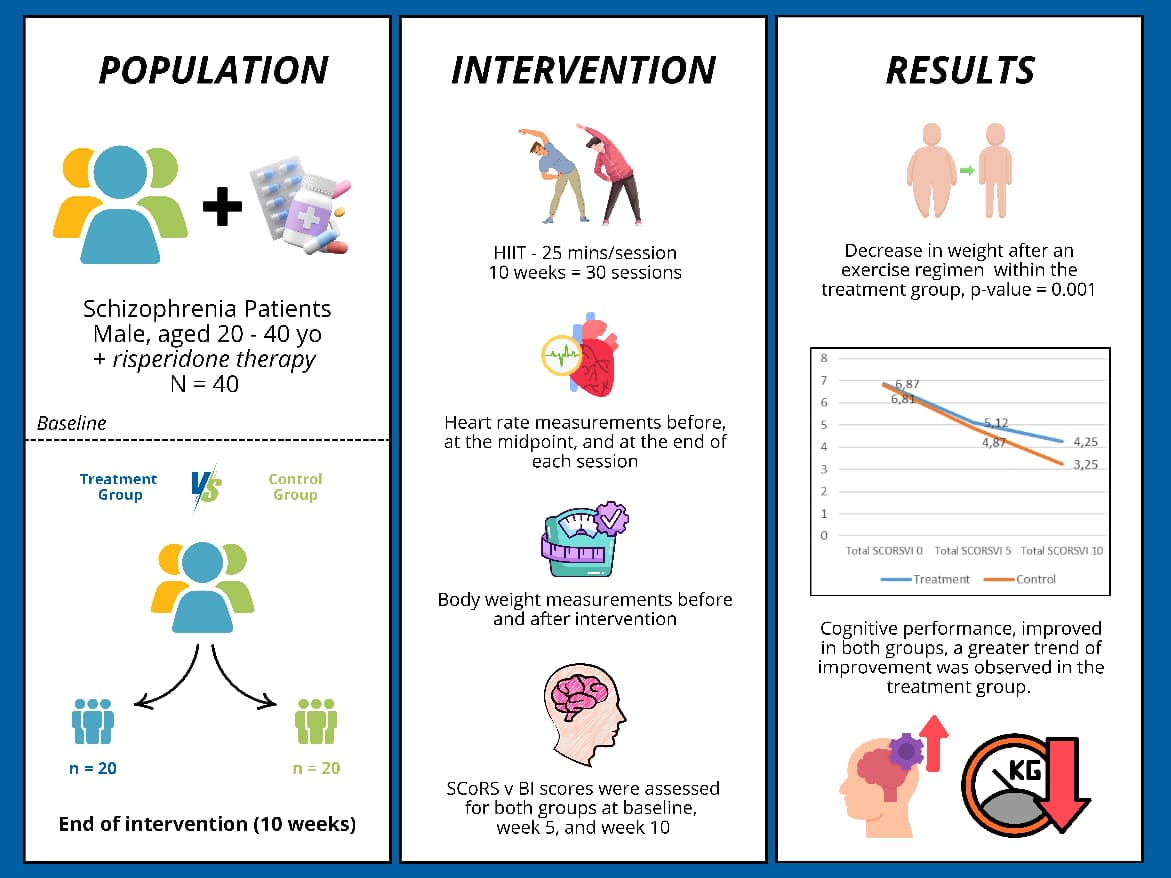
Vitamin A is an essential nutrient to support the function of vision, growth, and immune system. Vitamin A cannot be synthesized in the body hence must be obtained through foods or supplements. However, oral administration of vitamin A is often hindered by poor absorption due to its hydrophobic nature and by its easily degradable nature by light and oxygen, particularly at high temperature. This study aimed to prepare a self-assembly vitamin A nanoemulsion with a high loading capacity to improve vitamin A absorption accross intestinal mucosa and to slow down its degradation process. The nanoemulsion was composed by glyceryl monooleate, Cremophor RH-40, and PEG 400 (1:8:1), then titrated with aqueous phase. The nanoemulsion characterization included globule size evaluation, size distribution, zeta potential, globule morphology, entrapment efficiency, physical and chemical stabilities, and ex vivo penetration test on New Zealand albino rabbit intestines. The vitamin A nanoemulsion was found to form transparent and nano-sized emulsions even when loaded with 16.67% vitamin A. The formula also produced 58.1±2.0 nm spherical globules with -0.69 mV zeta potential. Entrapment efficiency of vitamin A in the nanoemulsion was higher than 95%. The nanoemulsion shows stable after storage for 10 days at room temperature, as well as able to increase penetration rate compared to free vitamin A. Taken together, our established vitamin A nanoemulsion has a good stability and was proved to increase vitamin A absorption through intestinal mucosa while simultaneously decreased the vitamin A degradation rate.
Gao, L., Zhang, D., & Chen, M. (2008). Drug nanocrystals for formulation of poorly soluble drugs and its application as potential
drug delivery system. J Nanopart Res, 10(5), 851–852.
Imdad, A., Herzer, K., Mayo-Wilson, E., Yakoob, M. Y., & Bhutta, Z. A. (2010) Vitamin a supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. The Cochrane Collaboration and published in The Cochrane Library, Issue 12. doi:
1002/14651858.CD008524.pub2
Kyatanwar, A. U., Jadhav, K. R., & Kadam, V. J. (2010). Self micro-emulsifying drug delivery system (SMEDDS): Review. J Pharm Res,
(1): 75-83.
Kowalska, M., Ziomek, M,. & Zbikowska, A. (2015). Stability of cosmetic emulsion containing different amount of hemp oil. Int J Cosmet Sci, 37(4): 408 – 416.
Rao, S. V. R., & Shao, J. (2008). Selfnanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs: I. Formulation Development, Int J Pharm, 362(2-3), 7-8.
Rasaputri, D. H. (2010). Pengembangan SelfNanoemulsifying drug delivery system (SNEDDS) untuk penghantaran oral interferon alfa 2b: Formulasi, karakterisasi dan uji stabilitas fisik (Thesis). Sekolah
Farmasi ITB, Bandung.
Sauvant, P., Cansell, M., Sassi, A. H., & Atgie, C. (2011). Vitamin a enrichment: Caution with encapsulation strategies used for food
applications, Food Res Int, 46(2), 469-479. https://doi.org/10.1016/j.foodres.2011.09.025
Sumardjo, D. (2009). Pengantar kimia buku panduan kuliah mahasiswa kedokteran dan program strata I Fakultas Bioeksakta,
Jakarta: EGC.
Taha, E. I., Al-Saidan, S., Samy, A. M., & Khan, M. A. (2009). Preparation and in vitro characterization of self-nanoemulsified drug
delivery system (SNEDDS) of all-transretinol acetate, Int J Pharm, 285(1-2), 109-113.
Ujhelyi, Z., Vecsernyes, M., Feher, P., Kosa, D., Arany P., Nemes D., ... Bacskay I. (2018) Physico-chemical characterization of selfemulsifying drug delivery systems. Drug Discov Today, 27: 81–86. https://doi.org/10.1016/j.ddtec.2018.06.005
Zhang, G., Meng, F., Guo, Z., Guo, T., Peng, H., Xiao, J., Liu, B., ... Zhang, J. (2018). Enhanced stability of vitamin A palmitate
microencapsulated by γ-cyclodextrin metalorganic frameworks, J Microen, 35(3): 249–258.
Zhao, L., Wei, Y., Huang, Y., He, B., Zhou, Y., & Fu J. (2013) Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine, 8(1): 3769–3779.
- MEDIA GIZI INDONESIA Journal is the copyright owner of all materials published on this website.
- The formal legal provisions for access to digital articles of this electronic journal are subject to the terms of the Creative Commons Attribution-NonCommercial-ShareAlike license (CC BY-NC-SA 4.0), which means that MEDIA GIZI INDONESIA Journal and readers reserve the right to save, transmit media / format, manage in database, maintain, and publish articles as long as it continues to include the name of the Author.
- Printed and published print and electronic manuscripts are open access for educational, research and library purposes. In addition to these objectives, the editorial board shall not be liable for violations of copyright law.


2.png)